Metalloenzymes in natural product biosynthetic pathways
Katherine S.
Ryan
a and
Catherine L.
Drennan
b
aDepartment of Chemistry, The University of British Columbia, 2036 Main Mall, Vancouver, BC, Canada V6T 1Z1. E-mail: ksryan@chem.ubc.ca
bDepartments of Biology and Chemistry and the Howard Hughes Medical Institute, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. E-mail: cdrennan@mit.edu
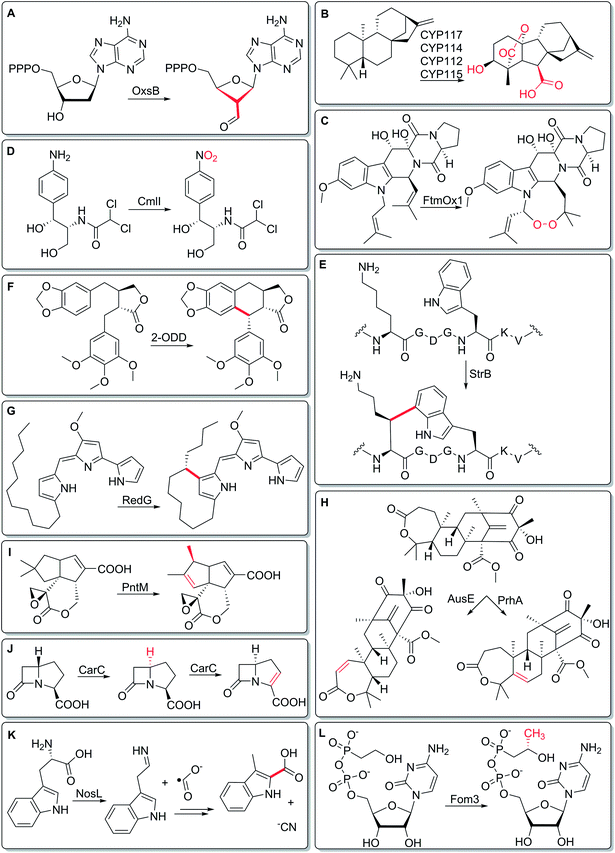
In this two-part themed issue on Metalloenzymes in Natural Product Biosynthetic Pathways, we bring together articles by experts from the worlds of biosynthesis and enzymology, who review enzymes that catalyze some of the most challenging chemistry in natural product biosynthesis. These reactions range from the impressive, yet expected – stereospecific hydroxylation of sp3-hybridized carbons, for example – to the utterly surprising, like the ring-contraction pathway in oxetanocin biosynthesis (Fig. 1A).1 Specifically, contributors to this issue focus on iron-containing enzymes. Iron is used as part of a heme cofactor in cytochrome P450 reactions, and as non-heme iron in Fe(II)/2-oxoglutarate-dependent enzymes. For radical SAM enzymes, a [4Fe–4S] cluster is required to generate the powerful 5′-deoxyadenosyl radical, which is key to catalysis. Rieske oxidases incorporate iron in [2Fe–2S] clusters, while non-heme diiron oxygenases chelate the metal directly via amino-acid residues. Across eleven articles, contributors to this themed issue on Metalloenzymes in Natural Product Biosynthetic Pathways highlight the versatility of iron cofactors in enzymes. Here, we provide a brief preview of this enzymatic diversity.
Metalloenzymes are perhaps best known for facilitating oxidative transformations that would be challenging using purely organic cofactors. For example, cytochrome P450s are classically known for installing hydroxyl groups at unactivated carbons (J. J. De Voss, M. J. Cryle et al., DOI: 10.1039/C7NP00063D), and recent work highlights the incredible biosynthetic pathway to gibberellins in rhizobial bacteria, involving multiple P450s, each catalyzing oxidative transformations (Fig. 1B).2 Another group of such oxidative enzymes is Fe(II)/2-oxoglutarate-dependent enzymes (X. Liu, P. Liu et al., DOI: 10.1039/C7NP00067G), whose repertoire includes FtmOx1-catalyzed endoperoxide formation (Fig. 1C).3 Diiron monooxygenases have also recently emerged as a powerful group of oxidative biosynthetic enzymes (J. D. Lipscomb et al., DOI: 10.1039/C7NP00061H), catalyzing reactions including arylamine oxidation (Fig. 1D), where a peroxo-diferric intermediate acts twice – first as a nucleophilic oxidant, then as an electrophilic oxidant.4
Oxidative carbon–carbon bond forming reactions also play a key part in building chemical complexity, and radical SAM enzymes can play a key role in mediating such reactions (K. Yokoyama and E. A. Lilla, DOI: 10.1039/C8NP00006A). For example, in the biosynthesis of streptide, a radical SAM enzyme forms a Lys–Trp crosslink, uniting an unactivated carbon with the aromatic indole (Fig. 1E).5 Similarly, the mayapple polyphenol podophyllotoxin pathway involves an intramolecular C–C bond forming reaction carried out by an Fe(II)/2-oxoglutarate-dependent enzyme (Fig. 1F),6 showcasing the emerging roles of these enzymes in plant biosynthetic pathways (J. M. Hagel and P. J. Facchini, DOI: 10.1039/C7NP00060J). Intramolecular oxidative cyclizations are also catalyzed by Rieske oxygenases (L. M. Alkhalaf, G. L. Challis et al., DOI: 10.1039/C8NP00004B), for example, RedG-mediated coupling of a saturated alkyl chain to a pyrrole in the formation of streptorubin B (Fig. 1G).7
Another major role of metalloenzymes in biosynthesis is functionalization of unactivated carbons at a late stage in biosynthetic pathways. For example, Fe(II)/2-oxoglutarate-dependent enzymes from fungal meroterpenoid pathways (Y. Matsuda, I. Abe et al., DOI: 10.1039/C7NP00055C) can specifically react with distinct sites of a complex terpenoid, as exhibited by the regiospecific activity of PrhA and AusE (Fig. 1H).8 Cytochrome P450s, whose distribution in Streptomyces biosynthetic pathways is highlighted by Shen and coworkers (B. Shen et al., DOI: 10.1039/C7NP00034K), often intercept late-stage intermediates, and can catalyze unusual reactions like the oxidative rearrangement in pentalenolactone biosynthesis (Fig. 1I).9 Indeed, the use of natural and engineered P450s to functionalize unactivated carbons at a late stage in total synthesis campaigns has been highlighted in several papers.10,11
But metal-catalyzed reactions need not give oxidative outcomes. For example, in fungal biosynthesis of β-lactams (C. J. Schofield, C. T. Lohans et al., DOI: 10.1039/C8NP00002F), the Fe(II)/2-oxoglutarate-dependent enzyme CarC exhibits a two-faced nature by catalyzing two different reactions: the first reaction is an epimerization12 and the second reaction is a desaturation (Fig. 1J). Hung-wen Liu, who writes about radical SAM reactions (M. W. Ruszczycky, H.-W. Liu et al., DOI: 10.1039/C7NP00058H), provides his perspective on why this enzyme family demonstrates such a diversity of reactivity, including both oxidative and redox–neutral reactions. He also points out some of the more surprising recent findings with regards to enzyme mechanisms, including that of tryptophan lyase NosL (Fig. 1K).13 Along the lines of unusual chemistry, Susan Wang (DOI: 10.1039/C7NP00059F) reviews cobalamin-dependent radical SAM enzymes, a subset of the 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-membered radical SAM enzyme superfamily, known for catalyzing unprecedented methylation reactions such as the one performed by Fom3 in fosfomycin biosynthesis (Fig. 1L).14,15
000-membered radical SAM enzyme superfamily, known for catalyzing unprecedented methylation reactions such as the one performed by Fom3 in fosfomycin biosynthesis (Fig. 1L).14,15
Altogether, the work highlighted in this collection emphasizes the striking variety of metalloenzyme-catalyzed transformations that build complexity. These articles also feature impressive experimental work, ranging from high-resolution X-ray crystallographic studies, to detailed spectroscopic work, to stoichiometric investigations of reaction outcomes, and highlight potential applications of this research for making new molecules and developing new biocatalysts. Indeed, all these experts agree: metalloenzymes in natural product biosynthesis are a rich source for new catalysts, and we have yet to fully realize the breadth of metalloenzyme-catalyzed chemistry.
References
- J. Bridwell-Rabb, A. Zhong, H. G. Sun, C. L. Drennan and H.-W. Liu, Nature, 2017, 544, 322–326 CrossRef PubMed.
- R. S. Nett, M. Montanares, A. Marcassa, X. Lu, R. Nagel, T. C. Charles, P. Hedden, M. C. Rojas and R. J. Peters, Nat. Chem. Biol., 2017, 13, 69–74 CrossRef PubMed.
- W. Yan, H. Song, F. Song, Y. Guo, C.-H. Wu, A. S. Her, Y. Pu, S. Wang, N. Naowarojna, A. Weitz, M. P. Hendrich, C. E. Costello, L. Zhang, P. Liu and Y. J. Zhang, Nature, 2015, 527, 539–543 CrossRef PubMed.
- A. J. Jasniewski, A. J. Komor, J. D. Lipscomb and L. Que, J. Am. Chem. Soc., 2017, 139, 10472–10485 CrossRef PubMed.
- K. R. Schramma, L. B. Bushin and M. R. Seyedsayamdost, Nat. Chem., 2015, 7, 431–437 CrossRef PubMed.
- W. Lau and E. S. Sattely, Science, 2015, 349, 1224–1228 CrossRef PubMed.
- D. M. Withall, S. W. Haynes and G. L. Challis, J. Am. Chem. Soc., 2015, 137, 7889–7897 CrossRef PubMed.
- Y. Nakashima, T. Mori, H. Nakamura, T. Awakawa, S. Hoshino, M. Senda, T. Senda and I. Abe, Nat. Commun., 2018, 9(1), 104 CrossRef PubMed.
- L. Duan, G. Jogl and D. E. Cane, J. Am. Chem. Soc., 2016, 138, 12678–12689 CrossRef PubMed.
- A. N. Lowell, M. D. DeMars, S. T. Slocum, F. Yu, K. Anand, J. A. Chemler, N. Korakavi, J. K. Priessnitz, S. R. Park, A. A. Koch, P. J. Schultz and D. H. Sherman, J. Am. Chem. Soc., 2017, 139, 7913–7920 CrossRef PubMed.
- S. A. Loskot, D. K. Romney, F. H. Arnold and B. M. Stoltz, J. Am. Chem. Soc., 2017, 139, 10196–10199 CrossRef PubMed.
- W. Chang, Y. Guo, C. Wang, S. E. Butch, A. C. Rosenzweig, A. K. Boal, C. Krebs and J. M. Bollinger, Science, 2014, 343, 1140–1144 CrossRef PubMed.
- D. M. Bhandari, D. Fedoseyenko and T. P. Begley, J. Am. Chem. Soc., 2018, 140, 542–545 CrossRef PubMed.
- S. Sato, F. Kudo, T. Kuzuyama, F. Hammerschmidt and T. Eguchi, Biochemistry, 2018 DOI:10.1021/acs.biochem.8b00614.
- M. I. McLaughlin and W. A. van der Donk, Biochemistry, 2018 DOI:10.1021/acs.biochem.8b00616.
| This journal is © The Royal Society of Chemistry 2018 |