Direct synthesis of diethyl carbonate from ethanol and carbon dioxide over ceria catalysts
Abstract
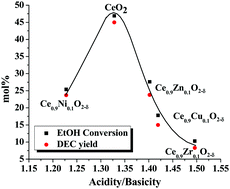
Direct synthesis of diethyl carbonate (DEC) by carboxylation of ethanol with CO2 was investigated over ceria catalysts. 2-Cyanopyridine (2-CP) was used for trapping water formed in the reaction and to shift the equilibrium towards the product side. An optimal dependence (“volcanic plot”) of the catalytic activity on the acidity/basicity molar ratio was observed. “Neat” ceria (procured from Daiichi Kigenso Kagaku Kogyo Co. Ltd, Japan) showed higher catalytic activity than metal incorporated ceria catalysts. CeO2 had the right kind of acidity/basicity ratio to activate ethanol and CO2 simultaneously, yielding DEC. The catalyst was reusable. The yield of DEC obtained in this study using the commercial catalyst was higher than that reported by others using ceria catalysts prepared by other methods. Under optimum conditions (ethanol : 2-CP molar ratio = 2 : 1, catalyst = 2.17 wt% with respect to ethanol, CO2 pressure = 40 bar, reaction temperature = 150 °C and reaction time = 3 h) in a batch reaction, a DEC yield as high as 45 mol% (i.e., 38.7 mmol mmol−1 of CeO2) was obtained.


 Please wait while we load your content...
Please wait while we load your content...