A novel near-infrared fluorescent probe with a “donor–π–acceptor” type structure and its application in the selective detection of cysteine in living cells†
Haojia
Hong
a,
Lei
Shi
 *a,
Junzhe
Huang
a,
Chang
Peng
*b,
Sheng
Yang
*a,
Junzhe
Huang
a,
Chang
Peng
*b,
Sheng
Yang
 c,
Guang
Shao
c,
Guang
Shao
 d and
Shengzhao
Gong
*a
d and
Shengzhao
Gong
*a
aSchool of Chemical Engineering and Technology, Guangdong Industry Polytechnic, Guangzhou, Guangdong, 510300, P. R. China. E-mail: 2016103059@gdip.edu.cn; 1996103022@gdip.edu.cn
bCollege of Science, Hunan Agricultural University, Changsha, Hunan 411105, P. R. China. E-mail: pch1026@126.com
cHunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, School of Chemistry and Biological Engineering, Changsha University of Science and Technology, Changsha, Hunan 410114, P. R. China
dSchool of Chemistry, Sun Yat-sen University, Guangzhou, Guangdong, 510275, P. R. China
First published on 12th November 2018
Abstract
The development of imaging-suitable fluorescent probes based on fluorogen scaffolds with near-infrared emission is crucial for monitoring cysteine (Cys) in biological systems. Herein, we synthesized a novel NIR dye based on a “donor–π–acceptor” structure which exhibits good Stokes shifts, satisfactory quantum yields, long wavelength excitation and excellent photostability, and a Cys-responsive NIR probe was constructed by decoration with methacrylate. The designed probe possesses great sensitivity and selectivity for detection of Cys. Moreover, it has been successfully applied in detecting Cys in living cells, indicating its great potential value for biological applications.
1. Introduction
Biothiols, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), have attracted much attention in recent years due to their vital functions in defending against oxidants and maintaining biological systems.1–3 Among them, Cys plays vital roles in a wide range of physiological processes such as protein synthesis, detoxification and metabolism. However, its abnormal levels in living cells would cause retardation in growth, skin lesions, liver damage, edema, lethargy, and Alzheimer's and cardiovascular diseases.4–8 So developing a sensitive and convenient method for selective recognition of Cys in living cells and biological systems is indispensable.Fluorescence sensing is one of the most popular analytical methods for detecting biological thiols.3,9,10 Unlike the probes with fluorescent signals within the ultraviolet or visible-light range, the near-infrared (NIR) fluorescent probes are quite suitable for biological imaging applications because of their great technical advantages, such as less damage to biological samples, deeper tissue penetration, and lower interference from the background.11–13
To date, several NIR fluorescent probes for Cys detection have been reported, but most of them are still based on squaraine,14 cyanine dyes,15 naphthofluorescein,6 or dicyano-methylene-benzopyran.16 These probes cannot be effectively utilized due to some of their disadvantages, such as long response times, small Stokes shifts, low quantum yields, low sensitivity and/or complex synthesis. Therefore, it is desirable and necessary to develop novel NIR fluorescent probes for detection and bioanalysis of Cys.
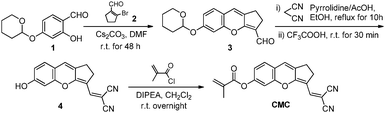
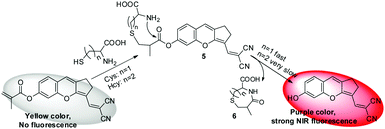
In this study, we synthesized a novel NIR fluorescent fluorophore which was based on a “donor–π–acceptor” structure and possessed good optical performances, and a new fluorescent probe CMC (Scheme 1) was synthesized for detection of Cys. The probe could selectively react with Cys over other biological analytes, and the concentration of Cys could be quantitatively determined by UV-vis absorption or fluorescent signals. More importantly, we have successfully applied this probe in detecting Cys in living cells.
2. Experimental
2.1 Materials and instruments
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were acquired using a Bruker AVANCE III spectrometer. High resolution mass spectrometry (HRMS) was performed using an ESI-Q-TOF mass spectrometer (Bruker Daltonics, ESI-Q-TOFmaXis 4G). UV-vis spectra were measured using a Hitachi U-4100 spectrophotometer. Quartz cuvettes with 1 cm path length and 3 mL volume were used for all measurements. An Edinburgh F35 luminescence spectrophotometer was used to measure fluorescence. A Mettler Toledo pH meter was employed to determine the pH values. Fluorescence images of cells were obtained using an Olympus FV1000-MPE multiphoton laser scanning confocal microscope. Twice-distilled water was used for all experiments. All reagents were used without further purification.2.2 Synthetic procedures and methods
Compound 1 was obtained as colorless oil according to a reported procedure,17 and the crude product of compound 2 was prepared according to the literature18,19 and used directly in the next step without further purification.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to yield compound 3 as dark green solids (1.03 g, 49%). 1H NMR (400 MHz, CDCl3) δ 10.53 (s, 1H), 7.08 (d, J = 8.8 Hz, 1H), 6.93 (d, J = 2.4 Hz, 1H), 6.83 (dd, J = 8.4 Hz & 2.4 Hz, 1H), 6.62 (s, J = 3.2 Hz, 1H), 5.47 (t, 1H), 3.93–3.86 (m, 1H), 3.69–3.64 (m, 1H), 2.82–2.89 (m, 2H), 2.81–2.71 (m, 4H), 1.93–1.86 (m, 2H), 1.61–1.76 (m, 2H). 13C NMR (100 MHz, d6-DMSO) δ 182.9, 163.0, 157.5, 151.8, 137.1, 127.7, 122.3, 115.7, 115.6, 113.3, 103.7, 95.8, 61.5, 29.5, 24.5, 23.6, 23.3, 18.3. ESI-HRMS m/z: [compound 3 + H]+ calcd for C18H18O4 299.13, found 299.1277.
1, v/v) to yield compound 3 as dark green solids (1.03 g, 49%). 1H NMR (400 MHz, CDCl3) δ 10.53 (s, 1H), 7.08 (d, J = 8.8 Hz, 1H), 6.93 (d, J = 2.4 Hz, 1H), 6.83 (dd, J = 8.4 Hz & 2.4 Hz, 1H), 6.62 (s, J = 3.2 Hz, 1H), 5.47 (t, 1H), 3.93–3.86 (m, 1H), 3.69–3.64 (m, 1H), 2.82–2.89 (m, 2H), 2.81–2.71 (m, 4H), 1.93–1.86 (m, 2H), 1.61–1.76 (m, 2H). 13C NMR (100 MHz, d6-DMSO) δ 182.9, 163.0, 157.5, 151.8, 137.1, 127.7, 122.3, 115.7, 115.6, 113.3, 103.7, 95.8, 61.5, 29.5, 24.5, 23.6, 23.3, 18.3. ESI-HRMS m/z: [compound 3 + H]+ calcd for C18H18O4 299.13, found 299.1277.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the mixture was refluxed for 12 h. Then, trifluoroacetic acid (0.3 mL) was added and the resulting solution was stirred for another 30 min at room temperature. After this, the mixture was concentrated in vacuo and the residue was purified by silica gel column chromatography (CH2Cl2/EtOAc, 10
1), and the mixture was refluxed for 12 h. Then, trifluoroacetic acid (0.3 mL) was added and the resulting solution was stirred for another 30 min at room temperature. After this, the mixture was concentrated in vacuo and the residue was purified by silica gel column chromatography (CH2Cl2/EtOAc, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to yield compound 4 as dark purple solids (209 mg, 66%). 1H NMR (400 MHz, d6-DMSO) δ 10.53 (s, 1H), 7.80 (s, 1H), 7.34 (d, J = 8.4 Hz, 1H), 7.23 (s, 1H), 6.79 (s, 1H), 6.75 (dd, J = 8.4 Hz & 1.6 Hz, 1H), 2.91–2.98 (m, 2H), 2.82–2.89 (m, 2H). 13C NMR (100 MHz, d6-DMSO) δ 164.8, 160.2, 153.0, 146.3, 134.0, 128.7, 127.6, 117.2, 116.1, 114.3, 113.8, 113.2, 102.7, 28.9, 24.7. ESI-HRMS m/z: [compound 4 + H]+ calcd for C16H10N2O2 263.08, found 263.0814.
1, v/v) to yield compound 4 as dark purple solids (209 mg, 66%). 1H NMR (400 MHz, d6-DMSO) δ 10.53 (s, 1H), 7.80 (s, 1H), 7.34 (d, J = 8.4 Hz, 1H), 7.23 (s, 1H), 6.79 (s, 1H), 6.75 (dd, J = 8.4 Hz & 1.6 Hz, 1H), 2.91–2.98 (m, 2H), 2.82–2.89 (m, 2H). 13C NMR (100 MHz, d6-DMSO) δ 164.8, 160.2, 153.0, 146.3, 134.0, 128.7, 127.6, 117.2, 116.1, 114.3, 113.8, 113.2, 102.7, 28.9, 24.7. ESI-HRMS m/z: [compound 4 + H]+ calcd for C16H10N2O2 263.08, found 263.0814.
2.3 General method for spectral detection
A stock solution of the probe CMC was prepared (1.00 mmol L−1) in DMF, and its test solution (10 μmol L−1) was prepared by diluting the stock solution with a mixture of PBS buffer/DMF (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at an indicated pH value. Then various analytes were added separately and incubated for 30 min, and the UV-vis absorption and fluorescence spectra of these solutions were recorded in a quartz cuvette of 1 cm optical path length. For fluorescence measurements, the excitation wavelength was set at 614 nm with a slit width of 0.5 × 2.5 nm.
1) at an indicated pH value. Then various analytes were added separately and incubated for 30 min, and the UV-vis absorption and fluorescence spectra of these solutions were recorded in a quartz cuvette of 1 cm optical path length. For fluorescence measurements, the excitation wavelength was set at 614 nm with a slit width of 0.5 × 2.5 nm.
2.4 Detection limit calculation
The detection limit for HClO was calculated using eqn (1):20| Detection limit = 3σ/k | (1) |
2.5 Cell imaging study
The cytotoxic effects of the probe CMC were analyzed by using a methyl thiazolyl tetrazolium (MTT) assay.21Before the fluorescence imaging experiments, HeLa cells were seeded on 14 mm glass coverslips and allowed to adhere for 24 h. Then, 10 μmol L−1 probe CMC was added to the medium of HeLa cells, and the cells were incubated for 30 min at 37 °C. After washing treatment of the cells with PBS buffer (3 times), fluorescence imaging was carried out. The fluorescence signal was collected at 645–690 nm with a semiconductor laser at 635 nm as the excitation light source.
3. Results and discussion
3.1 Design and synthesis
The synthetic route to probe CMC is shown in Scheme 1, and the structures of intermediate compounds and the probe were fully characterized by 1H NMR, 13C NMR and HR-MS (Fig. S1–S12, ESI†).In this study, the fluorophore of the probe (compound 4) was based on the “donor–π–acceptor” type fluorophore which included a malononitrile moiety as the electron-withdrawing group and a phenolic hydroxyl group as the electron-donating group. Compound 4 displayed good NIR optical performances, such as long wavelength excitation, near infrared emission, good Stokes shift (Δλ = 39 nm), satisfactory fluorescence quantum yield (Φ = 0.19 in a mixture of PBS/DMF) and excellent photostability (Fig. S13, S14 and Table S1, ESI†). Then a methacrylate moiety, as an efficient electron acceptor and a selective reaction site for Cys,22 was introduced to the structure of the fluorophore to obtain probe CMC. As a result, the fluorescence intensity of the fluorophore was severely weakened due to the photoinduced electron transfer (PET) effect. After the addition of Cys, its fluorescence would recover soon by the removal of the acrylate group through selective chemical reactions.
3.2 Spectroscopic properties of the probe CMC
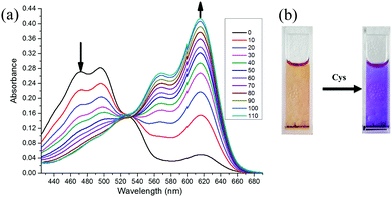
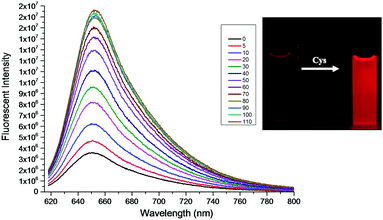
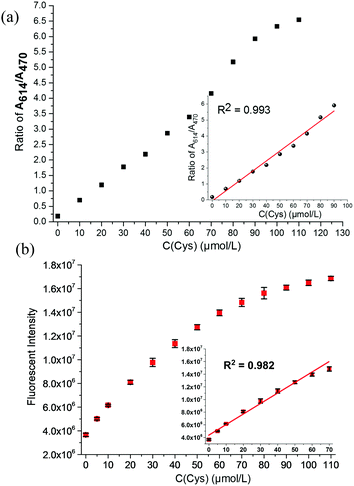
In order to verify the feasibility of the probe CMC, we first started to investigate its UV-vis absorption and fluorescence responses to Cys (0–110 μmol L−1) in the mixture of PBS/DMF (pH = 7.4, v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature. As shown in Fig. 1 and 2, the probe CMC emitted weak fluorescence in the absence of Cys. Soon after the addition of Cys, the probe's fluorescence band centred at 655 nm was gradually increased, and its UV-vis absorption band was shifted hugely from 480 nm to 614 nm with an apparent change of colour being visualized by the naked eye. Meanwhile, both the ratio of absorbance at 614 nm and 470 nm (A614nm/A470nm) and the fluorescence intensity at 655 nm (I655nm) were linearly dependent on the increased concentration of Cys (Fig. 3). There was good linearity obtained in the range of 0–80 μmol L−1 for Cys with a detection limit of 1.06 μmol L−1. In addition, the absolute fluorescence quantum yield (Φ) values were determined by using an integral sphere according to a previous report,23 and an obvious enhancement of the quantum yield was observed (Φ = 0.033 to 0.192, tested in the mixture of PBS buffer/DMF). These optical testing results manifested that the probe CMC could detect the Cys precisely and sensitively under physiological conditions.
1) at room temperature. As shown in Fig. 1 and 2, the probe CMC emitted weak fluorescence in the absence of Cys. Soon after the addition of Cys, the probe's fluorescence band centred at 655 nm was gradually increased, and its UV-vis absorption band was shifted hugely from 480 nm to 614 nm with an apparent change of colour being visualized by the naked eye. Meanwhile, both the ratio of absorbance at 614 nm and 470 nm (A614nm/A470nm) and the fluorescence intensity at 655 nm (I655nm) were linearly dependent on the increased concentration of Cys (Fig. 3). There was good linearity obtained in the range of 0–80 μmol L−1 for Cys with a detection limit of 1.06 μmol L−1. In addition, the absolute fluorescence quantum yield (Φ) values were determined by using an integral sphere according to a previous report,23 and an obvious enhancement of the quantum yield was observed (Φ = 0.033 to 0.192, tested in the mixture of PBS buffer/DMF). These optical testing results manifested that the probe CMC could detect the Cys precisely and sensitively under physiological conditions.
The pH effects on the fluorescence response for probe CMC to Cys were also investigated to evaluate the working pH ranges. As shown in Fig. S15 (ESI†), the probe displays significant responses under neutral or weak alkaline conditions, indicating that the probe CMC would work well in the physiological pH region.
3.3 Response time and selectivity study
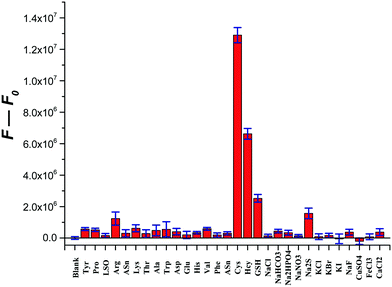
After testing the time-course fluorescence responses of the probe CMC to biothiols, it is evident from the results that the fluorescence intensity of the probe CMC increased quickly upon the addition of Cys and reached a plateau within 15 min (Fig. S16, ESI†). By comparison, only slight fluorescence enhancements could be observed in the same experiments on Hcy and GSH. Subsequently, the selectivity of the probe CMC among various amino acids and ions was also examined. Notably, only the addition of Cys caused a significant enhancement of the fluorescent signal and Hcy brought a moderate change of fluorescent signals, whereas the influence of other analytes was weak or negligible (Fig. 4). The results demonstrated that the probe CMC could detect Cys selectively and was very suitable for the real-time imaging of Cys in biological samples.3.4 Sensing mechanism
For the purpose of analysing the sensing mechanism of the probe CMC to Cys, 1H NMR, MS spectra and the TLC plate were employed.As shown in Fig. 5, after 10 min of adding various amounts of Cys in D2O, the characteristic chemical shifts of alkenyl hydrogen from 5.8 to 6.4 ppm disappeared, indicating the addition reaction proceeding between Cys and the alkene moiety of the probe CMC. And the chemical shifts of aromatic hydrogens moved to high fields and matched the chemical shifts of aromatic hydrogens in compound 4 very well. Meanwhile, the TLC plate also showed the chemical transformation from probe CMC to compound 4 (Fig. S19, ESI†). Moreover, the reaction mixture of Cys and probe was investigated by ESI-MS analysis. As shown in Fig. S20 (ESI†), the peaks at m/z 189.9, 189.9 and 280.8 indicated the formation of intermediate thioether product 5, cyclization product 6 and compound 4. All of these results clarified that the transformation from probe CMC to compound 4 was based on the strategy of the Michael addition and cyclization reaction, and this assumed mechanism shown in Scheme 2 showed a good agreement with a previous report.22
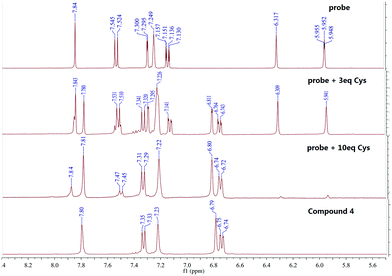 | ||
| Fig. 5 The comparisons of the 1H NMR spectra of probe CMC (d6-DMSO), compound 4 (d6-DMSO) and the reaction mixtures of probe (d6-DMSO) and Cys (D2O). | ||
3.5 Fluorescence imaging in living cells
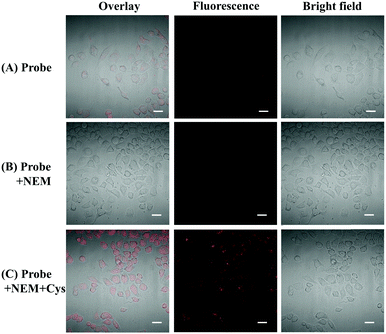
MTT assay was conducted to study the cytotoxicity of probe CMC to HeLa cells. As shown in Fig. S21 (ESI†), HeLa cells had a high survival rate (>95%) when the cells were incubated with 1–10 μmol L−1 of probe, indicating the low cytotoxicity of this probe.Afterwards, fluorescence imaging with probe CMC in living cells was carried out. As shown in Fig. 6A, slight fluorescence could be observed when HeLa cells were incubated with probe CMC for 30 min, indicating that the probe CMC was capable of penetrating into cells and detecting endogenous Cys by emitting NIR fluorescence. To validate the probe's specificity to cellular thiols, two control experiments were conducted. First, living cells were pretreated with a well-known thiol-blocking agent N-ethylmaleimide (NEM) for 60 min to deplete intracellular thiol species, and then incubated with the probe. As expected, no fluorescence was observed (Fig. 6B). By contrast, the cells were treated with NEM and Cys in turn, and then incubated with the probe. In this case, a marked time-dependent fluorescence enhancement was found (Fig. 6C). All of these imaging results clearly showed that the probe CMC was not responsive to intracellular compounds except Cys and this probe had great potential for biological applications.
4. Conclusions
In summary, we have developed a novel NIR fluorescent probe for the selective recognition of Cys. The structure of this probe is based on a “donor–π–acceptor” type structure and is synthesized through a few steps. The probe CMC takes advantage of long-wavelength light for excitation and displays near-infrared fluorescence with a good fluorescence quantum yield (Φ = 0.18 in PBS buffer/DMF). Moreover, it shows a remarkable and selective response to Cys with distinct UV-vis absorbance and fluorescent signal changes. Importantly, this probe has low cytotoxicity and good membrane permeability, and has been successfully applied to monitoring Cys in living cells. Therefore, this proposed NIR probe can be utilized well in biological applications for detecting Cys, and the structure of this fluorophore is worth exploring for detecting various biological analytes by replacement of recognition groups.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Research Project of Guangdong Education Department (2017GkQNCX003), the Foundation of Guangdong Industry Polytechnic (KYRC2017-0031), the National Natural Science Foundation (21606081), and the Science and Technology Planning Project of Guangzhou City (201607010349).Notes and references
- Z. A. Wood, E. Schröder, J. Robin Harris and L. B. Poole, Trends Biochem. Sci., 2003, 28, 32–40 CrossRef CAS.
- H. S. Jung, X. Chen, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019–6031 RSC.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- Y.-M. Go and D. P. Jones, Free Radical Biol. Med., 2011, 50, 495–509 CrossRef CAS.
- M. T. Heafield, S. Fearn, G. B. Steventon, R. H. Waring, A. C. Williams and S. G. Sturman, Neurosci. Lett., 1990, 110, 216–220 CrossRef CAS.
- S. Xue, S. Ding, Q. Zhai, H. Zhang and G. Feng, Biosens. Bioelectron., 2015, 68, 316–321 CrossRef CAS.
- S. Manna, P. Karmakar, S. S. Ali, U. N. Guria, R. Sarkar, P. Datta, D. Mandal and A. K. Mahapatra, New J. Chem., 2018, 42, 4951–4958 RSC.
- K. G. Reddie and K. S. Carroll, Curr. Opin. Chem. Biol., 2008, 12, 746–754 CrossRef CAS.
- Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192–270 CrossRef CAS.
- L.-Y. Niu, Y.-Z. Chen, H.-R. Zheng, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Soc. Rev., 2015, 44, 6143–6160 RSC.
- J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626–634 CrossRef CAS.
- V. Ntziachristos, C. Bremer and R. Weissleder, Eur. Radiol., 2003, 13, 195–208 Search PubMed.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- Q. Lin, Y. Huang, J. Fan, R. Wang and N. Fu, Talanta, 2013, 114, 66–72 CrossRef CAS.
- Y. Guo, X. Yang, L. Hakuna, A. Barve, J. O. Escobedo, M. Lowry and R. M. Strongin, Sensors, 2012, 12, 5940 CrossRef CAS.
- Y. Qi, Y. Huang, B. Li, F. Zeng and S. Wu, Anal. Chem., 2018, 90, 1014–1020 CrossRef CAS PubMed.
- K. H. Baek, R. Karki, E.-S. Lee, Y. Na and Y. Kwon, Bioorg. Chem., 2013, 51, 24–30 CrossRef CAS PubMed.
- O. M. J. Hsien, D. Sylvain, M. Mathieu, R. Anthony and R. Jean-Alexandre, Chem. – Asian J., 2017, 12, 936–946 CrossRef.
- A. Romieu and J.-A. Richard, Tetrahedron Lett., 2016, 57, 317–320 CrossRef CAS.
- J.-Y. Xie, C.-Y. Li, Y.-F. Li, J. Fei, F. Xu, J. Ou-Yang and J. Liu, Anal. Chem., 2016, 88, 9746–9752 CrossRef CAS.
- T. Rasheed, C. Li, F. Nabeel, M. Qi, Y. Zhang and C. Yu, New J. Chem., 2018, 42, 10940–10946 RSC.
- W.-W. Ma, M.-Y. Wang, D. Yin and X. Zhang, Sens. Actuators, B, 2017, 248, 332–337 CrossRef CAS.
- N. Hasebe, K. Suzuki, H. Horiuchi, H. Suzuki, T. Yoshihara, T. Okutsu and S. Tobita, Anal. Chem., 2015, 87, 2360–2366 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of chemical sensors and all emission and absorption data. See DOI: 10.1039/c8nj04006k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |