Curcumin nanoconjugate inhibits aggregation of N-terminal region (Aβ-16) of an amyloid beta peptide†
Abstract
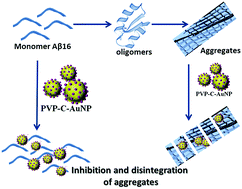
The present study describes a curcumin nanoformulation that inhibits amyloid beta (Aβ) aggregation. Curcumin is well known for its anti-carcinogenic, antioxidant and anti-inflammatory properties. Recent studies have shown that curcumin also possesses neuroprotective and cognitive-enhancing properties that may help to delay or prevent amyloid beta aggregation in Alzheimer's disease. However, due to the poor aqueous solubility and bioavailability of curcumin, its clinical applications are limited. To increase the bioavailability of curcumin, a highly stable and completely water soluble system consisting of a polymeric nanoparticle-encapsulated curcumin conjugate with gold nanoparticles decorated on the surface (PVP–C–AuNP) was formulated. The curcumin nano-conjugates were characterized by various physical, chemical and spectroscopic techniques including UV-visible spectroscopy, FT-IR spectroscopy, DLS and zeta potential analysis. The surface morphology of the conjugates was studied using TEM. Though the aggregation properties of full-length Aβ42 have been explored in detail, a shorter Aβ fragment from the N-terminal region of the peptide, Aβ1–16, was chosen for the current study as it demonstrates seeding properties for aggregate formation in the healthy brain. The aggregation kinetics of Aβ1–16 was studied using NMR spectroscopy and TEM. The fabricated curcumin nanoformulations were not only found to inhibit Aβ1–16 aggregation but they also disintegrated already formed aggregates.


 Please wait while we load your content...
Please wait while we load your content...