 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cinchona derivatives as sustainable and recyclable homogeneous organocatalysts for aza-Markovnikov addition†
Sándor
Nagy
 ,
Zsuzsanna
Fehér
,
Péter
Kisszékelyi
,
Zsuzsanna
Fehér
,
Péter
Kisszékelyi
 ,
Péter
Huszthy
,
Péter
Huszthy
 and
József
Kupai
and
József
Kupai
 *
*
Department of Organic Chemistry and Technology, Budapest University of Technology and Economics, P.O. Box 91, Budapest, H-1521, Hungary. E-mail: jkupai@mail.bme.hu; Tel: 0036-1463-2229
First published on 12th April 2018
Abstract
Three cinchona derivatives have shown remarkable activity to catalyze the aza-Markovnikov addition reaction of N-heterocycles to vinyl esters. The synthesis of aza-Markovnikov adducts possessing valuable biological activity was thoroughly optimized. By studying the ratio of the starting materials, bases and solvents, we achieved a new and efficient protocol, which could be performed under mild conditions with a small excess of vinyl ester affording products with excellent yields and high regioselectivity. This optimization reduced Sheldon's E-factor of the reaction by 42%. Furthermore, membrane separation for catalyst recycling was assessed to further improve the sustainability of the synthesis.
Introduction
Catalytic transformations play an increasingly important role in organic chemistry today, both in academic laboratories and in industries. Organocatalysts, enzymes and homogeneous metal catalysts are expensive, therefore new strategies have to be developed to obtain maximum catalyst performance with regards to selectivity and turnover numbers. Membrane separation in organic media is a sustainable separation technology to achieve these goals, along with process intensification and a cleaner product stream.Cinchona alkaloids, originally isolated from the bark of Cinchona trees, are amongst the most well-known natural products with exceptional medical history and their derivatives have emerged as powerful organocatalysts, which are reported in several reviews1–4 and recently in books.5,6 The widespread usage of cinchona alkaloids has been attributed to their non-toxicity, ease of use, stability, cost effectiveness, recyclability, and practical utilization in industries.7–10
Aza-Markovnikov addition is a useful nitrogen–carbon bond-forming reaction, in particular, for the synthesis of bioactive N-heterocycle derivatives. 1-(N-Heterocycle) alkyl esters, which could be obtained by this reaction, possess valuable biological properties11 and can act as acaricides (A),12,13 antitumor drugs (B),14 (H+–K+)-ATPase inhibitors (C)15 and are also used to treat gout and certain types of kidney stones (D) (see Fig. 1).16 Consequently, many researchers have focused on developing new methodologies for the synthesis of 1-(N-heterocycle) alkyl esters. However, most of the reported synthetic protocols are associated with the use of harsh chemical conditions, in which, bases, acids and intense heating are usually applied to promote the reaction. In many cases, yield and selectivity are far from satisfactory due to several side reactions.
Many efforts have been made regarding green syntheses. In the mid-2000s, a new enzymatic strategy to perform Markovnikov addition was developed with the use of penicillin G acylase as catalyst.16,17 Later, Lin and his co-workers applied K3PO4 as a mild base,11 and recently Chen and his co-workers used ionic liquids as reaction media and catalysts.18 However, it is unsustainable to scale up this modified synthesis due to the high excess of vinyl ester and the application of problematic solvents such as DMF.
A serious practical problem with homogeneous catalysis is the separation of reactants and products from the catalyst, which are all in the same phase. The applicability of membrane-based separation for the recovery of homogeneous organocatalysts was explored. Membrane-based separation in organic media is a green technology that allows size-exclusion based separation of solutes in the range of 50 and 2000 g mol−1 by applying a pressure gradient.19 Recent development in this field resulted in membranes, which can withstand aggressive solvents and exhibit high flux, while quasi completely rejecting relatively small solutes at the lower end of the nanofiltration range.20,21 Homogeneous catalyst recovery using membranes is an emerging field due to its mild operating conditions, low cost and easy implementation in continuous processing.22,23
Here, we report a new application of cinchona alkaloids in the catalysis of aza-Markovnikov addition. The addition of N-heterocycles (imidazole, benzimidazole, pyrazole or 1,2,3-triazole) to vinyl esters (vinyl acetate or vinyl 4-tert-butylbenzoate) was studied and a mechanism is suggested. Our aim was to develop a new method for efficient synthesis of biologically active aza-Markovnikov adducts, avoiding tedious and expensive repeated purifications and using homogeneous catalysts, which could be easily recycled after the reaction. Furthermore, our new synthesis of aza-Markovnikov adducts was evaluated through Sheldon's E-factor.24,25
Results and discussion
To turn the aza-Markovnikov reaction more eco-friendly, two model reactions were chosen (Scheme 1) using N-heterocycles (1 and 2) as substrates and vinyl acetate (3) as a reagent resulting in two aza-Markovnikov adducts (4 and 5).At first, it was observed that decreasing the reaction temperature (from 50 °C to 25 °C) had no significant effect on the yield of the reaction, (see Table 1, entries 1, 2 and 8, 9) and aza-Markovnikov addition could proceed at room temperature. Then, the influence of solvent, the molar ratio of vinyl acetate and catalyst (K3PO4) to N-heterocycles was investigated. DMF was replaced by a greener alternative,26–28 acetonitrile (see Table 1), providing an easier work-up process due to its lower boiling point. According to the recent critical review by Byrne et al.29 on solvent selection, substitution of DMF is required, and acetonitrile is a suitable replacement. The amounts of catalyst and reagent were also decreased (see Table 1).
| Entry | Reagent | Solvent | Temperature [°C] | Equivalent of catalyst | Equivalent of vinyl ester | Yieldb [%] |
|---|---|---|---|---|---|---|
| a The aza-Markovnikov addition reaction of N-heterocycle 1 or 2 (0.6 mmol) and vinyl acetate 3, with the catalyst K3PO4 in 1.2 mL of solvent after 48 h. b Isolated yield of the purified material. | ||||||
| 1 | 1 | DMF | 50 | 0.3 | 8 | 65 |
| 2 | 1 | DMF | 25 | 0.3 | 8 | 63 |
| 3 | 1 | DMF | 25 | 0.05 | 8 | 62 |
| 4 | 1 | DMF | 25 | 0.05 | 1.2 | 52 |
| 5 | 1 | MeCN | 25 | 0.3 | 8 | 63 |
| 6 | 1 | MeCN | 25 | 0.05 | 8 | 58 |
| 7 | 1 | MeCN | 25 | 0.05 | 1.2 | 48 |
| 8 | 2 | DMF | 50 | 0.3 | 8 | 61 |
| 9 | 2 | DMF | 25 | 0.3 | 8 | 57 |
| 10 | 2 | DMF | 25 | 0.05 | 8 | 55 |
| 11 | 2 | DMF | 25 | 0.05 | 1.2 | 50 |
| 12 | 2 | MeCN | 25 | 0.3 | 8 | 60 |
| 13 | 2 | MeCN | 25 | 0.05 | 8 | 55 |
| 14 | 2 | MeCN | 25 | 0.05 | 1.2 | 44 |
The optimized process was compared to previous references by means of Sheldon's E-factor. The optimization was carried out considering the load of all input materials. The results are summarized in Table 2, and the E-factor for each process is expressed as the mass ratio of waste to the desired aza-Markovnikov adduct.
| Entry | Reagent | E-factora | Ref. |
|---|---|---|---|
| a To achieve meaningful comparisons of different processes, solvent is generally excluded from the E-factor calculation. It is not possible to include the materials used for chromatographic purification in this comparison, since amounts of these materials are never reported in journal articles. | |||
| 1 | 1 | 2.51 | 11 |
| 2 | 1 | 3.05 | 30 |
| 3 | 1 | 1.46 | Our work |
| 4 | 2 | 2.26 | 11 |
| 5 | 2 | 2.52 | 30 |
| 6 | 2 | 1.58 | Our work |
As a result of the optimization, the yields were a bit lower (in the case of imidazole: from 65% to 48%, and in the case of benzimidazole: from 61% to 44%). As a positive result, the decreases of E-factor calculated over the aza-Markovnikov addition to imidazole and benzimidazole are 42% and 30%, respectively. Therefore our results suggest that this method is greener than the ones previously reported.11,30
Three cinchona catalysts (6–8, see Fig. 2) were also applied as homogeneous catalysts in aza-Markovnikov additions of these N-heterocycles to vinyl esters. Hydroquinine (6) is a commercially available versatile organocatalyst. The latter was converted into its amine derivative (7) after mesylation, azide formation and catalytic hydrogenation. Amine 7 was reacted with the condensation product of 3,5-bis(trifluoromethyl)aniline and dimethyl squarate to give a bifunctional cinchona-squaramide catalyst 8.31
Cinchona alkaloids are well-known asymmetric catalysts that give products with high enantiomeric excesses. In the beginning of our study, we tried to prepare aza-Markovnikov adducts enantioselectively. According to our latest experiments, enantiomeric excess is higher when reactions are performed at lower temperatures. Therefore, the cinchona-based organocatalysts were tested and compared to potassium phosphate first at 0 °C under the previously optimized circumstances in the aza-Markovnikov addition of four different N-heterocycles (1, 2, 9, 10, see Table 3) to vinyl acetate (3) or vinyl 4-tert-butylbenzoate (11). Mostly, the yields were lower when cinchona catalysts were used instead of potassium phosphate and the reactions gave racemic (ee lower than 5%) products (4, 5, 12–19, see Table 3).
a The aza-Markovnikov addition reaction of various N-heterocycles 1 or 2 or 9 or 10 (0.6 mmol) and vinyl esters 3 or 11 (0.72 mmol, 1.2 eq.), with catalysts K3PO4 or 6 or 7 or 8, in 1.2 mL of acetonitrile at 0 °C.
b Isolated yield of the purified material.
c After unsuccessful results were observed in the case of products 4 and 5, catalyst 8 was not applied in the other reactions.
d Starting from vinyl acetate (3) or vinyl 4-tert-butylbenzoate (11), and 1,2,3-triazole (10) two products (13 and 14) or (16 and 17) formed in about a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. 1 ratio.
|
|---|

|
The results suggest that the acyloyl part of the reagent has no influence on the reactivity: there was no significant difference between the aliphatic and aromatic reagents. By using cinchona catalysts 6–8, we achieved better yields only in the case of reactions with imidazole (1) and benzimidazole (2). Hence, we continued the optimization using N-heterocycles 1 and 2 and vinyl acetate (3) at elevated temperatures to produce aza-Markovnikov adducts 4 and 5 with higher yields.
Finally, cinchona-based organocatalysts 6–8 were applied at 25 °C and 50 °C, and the results were compared to those obtained using potassium phosphate. As shown in the results in Table 4, by applying cinchona amine 7, we obtained the aza-Markovnikov adducts with two times higher yields, than in the case when potassium phosphate was used. Consequently, based on our experimental results, the aza-Markovnikov reaction can be performed most environmentally friendly at 25 °C, using acetonitrile as a solvent and 5 mol% of cinchona amine 7. The high yield obtained by using cinchona amine 7, can be attributed to mechanistic reasons. The above experimental results, in accordance with the literature,32 suggest a mechanism for the aza-Markovnikov addition reaction catalyzed by cinchona amine 7 as shown in Scheme 2. In a similar manner to aminocatalysis,33 first the quinuclidine nitrogen of the cinchona amine 7 deprotonates the imidazole, then the primary amino group of the cinchona amine 7 forms an enamine-type intermediate with vinyl acetate (3). After that, the non-bonding electron pair of the deprotonated imidazole attacks the electron poor carbon atom of vinyl acetate (3). Finally, with the elimination of the product (4), the starting cinchona amine 7 is recovered.
| Yields (%) of 4 at: | Catalyst | Yields (%) of 5 at: | Catalyst | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K3PO4 | 6 | 7 | 8 | K3PO4 | 6 | 7 | 8 | ||
| a The aza-Markovnikov addition reaction of N-heterocycles 1 or 2 (0.6 mmol) and vinyl acetate 3, with catalysts K3PO4 or cinchona catalysts 6–8 in 1.2 mL of acetonitrile at different temperatures for 48 h. | |||||||||
| 0 °C | 35 | 32 | 48 | 0 | 0 °C | 31 | 29 | 41 | 4 |
| 25 °C | 48 | 35 | 95 | 0 | 25 °C | 44 | 39 | 92 | 4 |
| 50 °C | 91 | 57 | 98 | 77 | 50 °C | 89 | 60 | 96 | 74 |
Organic solvent nanofiltration (OSN) for recycling the cinchona catalysts 6–8 was explored. Commercial GMT-oNF and in-house fabricated PBI (poly[2,2′-(m-phenylene)-5,5′-bisbenzimidazole]) membranes were screened in acetonitrile at 10–30 bar pressure to determine their separation potential (Fig. 3). Efficient catalyst recovery requires as high catalyst rejection as possible, ideally 100%. The GMT-oNF-3 and PBI membranes at 30 bar showed the highest catalyst rejection of 98.3% and 99.1%, respectively. The latter membrane was selected for the purification process because of the lower product rejection (17%) compared to that of GMT-oNF-3. The rejection of vinyl acetate (3), resulting from the 0.2 molar excess, was found to be as low as 13%, which allows rapid purge from the system.
 | ||
| Fig. 3 Separation performance of the nanofiltration membranes in acetonitrile at 10–30 bar pressure. | ||
The single stage diafiltration allowed 99% product removal in 8.7 diavolumes at the cost of 8% catalyst lost (Fig. 4). The simplified membrane cascade developed by Kim et al. offers a sustainable approach to improve the catalyst recovery.34 Application of their process configuration resulted in a two-stage diafiltration cascade that requires about 10 diavolumes to achieve 99% product removal and at the same time the catalyst loss can be maintained as low as 1% (Fig. 4). The purity of the recycled cinchona catalyst 7 was confirmed by NMR, which confirmed that these catalysts do not degrade under the mild conditions applied during these reactions. The sustainability of the diafiltration can be further improved by in situ solvent recovery as recently demonstrated by Szekely et al.35,36
Conclusions
In summary, a series of reported (4, 5, 12) and novel (13–19) 1-(N-heterocycle) alkyl esters were synthesized and characterized. Aza-Markovnikov reactions were performed environmentally friendly at room temperature, by replacing undesirable dimethyl formamide with acetonitrile, and decreasing the amount of the catalyst (from 30% to 5%) and vinyl ester (from 8 equivalent to 1.2 equivalent). The Sheldon's E-factors calculated for the aza-Markovnikov addition to imidazole and benzimidazole were reduced by 42%, and 30%, respectively.Cinchona-based organocatalysts were synthesized and successfully applied in aza-Markovnikov addition as homogeneous catalysts. Using cinchona amine 7 as a catalyst, we obtained more than twice as high yield (92–95%) as that obtained using potassium phosphate. In the former case, a reaction mechanism was suggested.
The homogeneous catalytic implementation of aza-Markovnikov addition made this reaction more environmentally friendly by using OSN as its work up process. Due to the OSN technique, the applied catalysts were quasi completely recycled from the reaction mixture. The feasibility of membrane-based separation for catalyst recovery was demonstrated with potential to keep the catalyst loss below 1% using a two-stage cascade configuration.
Experimental – materials
GMT-oNF-1, GMT-oNF-2 and GMT-oNF-3 membranes were purchased from BORSIG Membrane Technology GmbH (Germany). 26 wt% polybenzimidazole dissolved in N,N-dimethylacetamide (DMAc) was purchased from PBI Performance Products Inc. (USA). Non-woven polypropylene fabric Novatexx 2471 was obtained from Freudenberg Filtration Technologies (Germany).Experimental – membrane screening and diafiltration
The polybenzimidazole (PBI) membrane was prepared from a 26 wt% dope solution on a non-woven support sheet using a casting knife set to a thickness of 100 μm at a temperature of 20 °C based on the literature procedure.37 The feed solution for the membrane screening comprised a mixture of catalyst, product, and substrate each of them in 0.1 g L−1 concentration in acetonitrile. The pressure range for the screening was 10–30 bar and the tests were carried out in a cross-flow nanofiltration rig (Fig. 5). The feed solution was recirculated for 24 h followed by collection of the samples from the permeate and the retentate streams. By definition, the rejection of a solute is the relative concentration decrease between the two sides of the membrane (eqn (1)): | (1) |
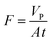 | (2) |
Experimental – preparation of organocatalysts and aza-Markovnikov reactions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (0.1% NH4HCO3) – acetonitrile (0.1% NH4HCO3 + 8% water)) in ESI mode. Enantioselectivities were determined by chiral HPLC using a Chiralpak column (256 nm, 20 °C, 2.0 mL min−1, hexane
water (0.1% NH4HCO3) – acetonitrile (0.1% NH4HCO3 + 8% water)) in ESI mode. Enantioselectivities were determined by chiral HPLC using a Chiralpak column (256 nm, 20 °C, 2.0 mL min−1, hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol = 4
isopropyl alcohol = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Voltage: 1.10 kV, m/z: 105–1000, scan speed: 1075 u s−1, DL temperature: 250 °C, Nebulizing gas flow: 1.5 L min−1, drying gas.
Flow: 15 L min−1. eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile: 0.1 v/v% formic acid (95
acetonitrile: 0.1 v/v% formic acid (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1.500 mL min−1). Elemental analyses were performed on a Vario EL III instrument (Element analyze Corp., Germany) in the Microanalytical Laboratory of the Department of Organic Chemistry, Institute for Chemistry, L. Eötvös University, Budapest, Hungary. The starting materials were purchased from Aldrich Chemical Company unless otherwise noted. Silica gel 60 F254 (Merck) plates were used for TLC. Silica gel 60 (70–230 mesh, Merck) was used for column chromatography. The ratios of solvents for the eluents are given in volumes (mL/mL). Evaporations were carried out under reduced pressure unless otherwise stated. The cinchona-based organocatalysts (7 and 8) were synthesized based on the experiments of Bae and co-workers.25
5, 1.500 mL min−1). Elemental analyses were performed on a Vario EL III instrument (Element analyze Corp., Germany) in the Microanalytical Laboratory of the Department of Organic Chemistry, Institute for Chemistry, L. Eötvös University, Budapest, Hungary. The starting materials were purchased from Aldrich Chemical Company unless otherwise noted. Silica gel 60 F254 (Merck) plates were used for TLC. Silica gel 60 (70–230 mesh, Merck) was used for column chromatography. The ratios of solvents for the eluents are given in volumes (mL/mL). Evaporations were carried out under reduced pressure unless otherwise stated. The cinchona-based organocatalysts (7 and 8) were synthesized based on the experiments of Bae and co-workers.25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (10
methanol (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as an eluent to give aza-Markovnikov adduct 4 as a pale yellow oil (yields can be seen in Tables 1, 3 and 4). Product 4 so obtained had the same spectroscopic data as those reported.38
1) mixture as an eluent to give aza-Markovnikov adduct 4 as a pale yellow oil (yields can be seen in Tables 1, 3 and 4). Product 4 so obtained had the same spectroscopic data as those reported.38
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (10
methanol (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as an eluent to give aza-Markovnikov adduct 5 as a pale yellow oil (yields can be seen in Tables 1, 3 and 4). Product 5 so obtained had the same spectroscopic data as those reported.38
1) mixture as an eluent to give aza-Markovnikov adduct 5 as a pale yellow oil (yields can be seen in Tables 1, 3 and 4). Product 5 so obtained had the same spectroscopic data as those reported.38
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (20
methanol (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as an eluent to give aza-Markovnikov adduct 12 as a pale yellow oil (yields can be seen in Table 3). Product 12 so obtained had the same spectroscopic data as those reported.38
1) mixture as an eluent to give aza-Markovnikov adduct 12 as a pale yellow oil (yields can be seen in Table 3). Product 12 so obtained had the same spectroscopic data as those reported.38
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (1
ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as an eluent to give aza-Markovnikov adduct 13 and 14 as pale yellow oils (yields can be seen in Table 3). TLC (SiO2 TLC; hexane
1) mixture as an eluent to give aza-Markovnikov adduct 13 and 14 as pale yellow oils (yields can be seen in Table 3). TLC (SiO2 TLC; hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 1
ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.62, UV).
1, Rf = 0.62, UV).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2946 (CH), 2094, 1751 (C
CH), 2946 (CH), 2094, 1751 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1485, 1444, 1371, 1301, 1284, 1220, 1196, 1071. δH(500 MHz; CDCl3; Me4Si) 1.96 (3 H, d, 3JH,H = 6.5 Hz, CCH3), 2.08 (3 H, s, CH3), 7.06 (1 H, q, JH,H 6.5, N–CH–O), 7.71 [1 H, s, TriazC(5)-H], 7.79 [1 H, s, TriazC(4)-H]; δC(75.5 MHz; CDCl3; Me4Si) 19.64 (CH–CH3 group), 20.79 (CH3), 77.64 (N–CH–O), 123.43 [TriazC(5)], 133.73 [TriazC(4)], 169.39 (COO); MS (ESI): exact mass calcd for C6H9N3O2: 155.16. Found m/z 156.200 (M+, 56.47%). Anal. calcd for C6H9N3O2: C, 46.45; H, 5.85; N, 27.08. Found: C, 46.26; H, 5.94; N, 27.07.
O), 1485, 1444, 1371, 1301, 1284, 1220, 1196, 1071. δH(500 MHz; CDCl3; Me4Si) 1.96 (3 H, d, 3JH,H = 6.5 Hz, CCH3), 2.08 (3 H, s, CH3), 7.06 (1 H, q, JH,H 6.5, N–CH–O), 7.71 [1 H, s, TriazC(5)-H], 7.79 [1 H, s, TriazC(4)-H]; δC(75.5 MHz; CDCl3; Me4Si) 19.64 (CH–CH3 group), 20.79 (CH3), 77.64 (N–CH–O), 123.43 [TriazC(5)], 133.73 [TriazC(4)], 169.39 (COO); MS (ESI): exact mass calcd for C6H9N3O2: 155.16. Found m/z 156.200 (M+, 56.47%). Anal. calcd for C6H9N3O2: C, 46.45; H, 5.85; N, 27.08. Found: C, 46.26; H, 5.94; N, 27.07.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2945 (CH), 1745 (C
CH), 2945 (CH), 1745 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1447, 1414, 1370, 1344, 1214, 1117, 1079, 1064. δH(300 MHz; CDCl3; Me4Si) 1.93 (3 H, d, JH,H 6.5, CCH3), 2.09 (3 H, s, CH3), 7.15 (1 H, q, JH,H 6.0, N–CH–O), 7.70 [2 H, s, TriazC-H]; δC(75.5 MHz; CDCl3; Me4Si) 19.28 (CH–CH3 group), 20.84 (CH3), 81.25 (N–CH–O), 135.17 [TriazC], 169.12 (COO); MS (ESI): exact mass calcd for C6H9N3O2: 155.16, found m/z 156.132 (M+, 44.78%). Anal. calcd for C6H9N3O2: C, 46.45; H, 5.85; N, 27.08. Found: C, 46.26; H, 5.94; N, 27.07.
O), 1447, 1414, 1370, 1344, 1214, 1117, 1079, 1064. δH(300 MHz; CDCl3; Me4Si) 1.93 (3 H, d, JH,H 6.5, CCH3), 2.09 (3 H, s, CH3), 7.15 (1 H, q, JH,H 6.0, N–CH–O), 7.70 [2 H, s, TriazC-H]; δC(75.5 MHz; CDCl3; Me4Si) 19.28 (CH–CH3 group), 20.84 (CH3), 81.25 (N–CH–O), 135.17 [TriazC], 169.12 (COO); MS (ESI): exact mass calcd for C6H9N3O2: 155.16, found m/z 156.132 (M+, 44.78%). Anal. calcd for C6H9N3O2: C, 46.45; H, 5.85; N, 27.08. Found: C, 46.26; H, 5.94; N, 27.07.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (20
methanol (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as an eluent to give aza-Markovnikov adduct 15 as a pale yellow oil (yields can be seen in Table 3). TLC (SiO2 TLC; dichloromethane
1) mixture as an eluent to give aza-Markovnikov adduct 15 as a pale yellow oil (yields can be seen in Table 3). TLC (SiO2 TLC; dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.48, UV). IR (neat) νmax/cm−1 3115, 2963 (CH), 2905 (CH), 2869 (CH), 1718 (C
1, Rf = 0.48, UV). IR (neat) νmax/cm−1 3115, 2963 (CH), 2905 (CH), 2869 (CH), 1718 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1608 (C
O), 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1493, 1261, 1223, 1188, 1071, 1033, 1014. δH(500 MHz; CDCl3; Me4Si) 1.35 (9 H, s, tBu CH3 groups), 1.95 (3 H, d, JH,H 6.5, CCH3), 6.99 (1 H, q, JH,H 6.0, N–CH–O), 7.10 [1 H, s, ImC(4)-H], 7.29 [1 H, s, ImC(5)-H], 7.48 and 7.95 (2 × 2H, AA′ BB′, JAB 8.5, Ph-H), 7.85 [s, 1 H, ImC(2)-H]; δC(125 MHz; CDCl3; Me4Si) 20.44 (CH–CH3 group), 31.06 (tBu CH3 groups), 35.18 (tBu C–CH3), 75.63 (N–CH–O), 116.85 [ImC(5)], 125.53 [PhC(3)], 126.08 [PhC(1)], 129.68 [ImC(4)], 129.74 [PhC(2)], 136.50 [ImC(2)], 157.61 [PhC(4)], 165.04 (COO); MS (ESI): exact mass calcd for C16H20N2O2: 272.15, found m/z 273.200 (M+, 100%). Anal. calcd for C16H20N2O2: C, 70.56; H, 7.40; N, 10.29. Found: C, 70.48; H, 7.59; N, 10.19.
C), 1493, 1261, 1223, 1188, 1071, 1033, 1014. δH(500 MHz; CDCl3; Me4Si) 1.35 (9 H, s, tBu CH3 groups), 1.95 (3 H, d, JH,H 6.5, CCH3), 6.99 (1 H, q, JH,H 6.0, N–CH–O), 7.10 [1 H, s, ImC(4)-H], 7.29 [1 H, s, ImC(5)-H], 7.48 and 7.95 (2 × 2H, AA′ BB′, JAB 8.5, Ph-H), 7.85 [s, 1 H, ImC(2)-H]; δC(125 MHz; CDCl3; Me4Si) 20.44 (CH–CH3 group), 31.06 (tBu CH3 groups), 35.18 (tBu C–CH3), 75.63 (N–CH–O), 116.85 [ImC(5)], 125.53 [PhC(3)], 126.08 [PhC(1)], 129.68 [ImC(4)], 129.74 [PhC(2)], 136.50 [ImC(2)], 157.61 [PhC(4)], 165.04 (COO); MS (ESI): exact mass calcd for C16H20N2O2: 272.15, found m/z 273.200 (M+, 100%). Anal. calcd for C16H20N2O2: C, 70.56; H, 7.40; N, 10.29. Found: C, 70.48; H, 7.59; N, 10.19.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (20
methanol (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as an eluent to give aza-Markovnikov adduct 16 as a pale yellow oil (yields can be seen in Table 4). TLC (SiO2 TLC; dichloromethane
1) mixture as an eluent to give aza-Markovnikov adduct 16 as a pale yellow oil (yields can be seen in Table 4). TLC (SiO2 TLC; dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.68, UV). IR (neat) νmax/cm−1 3058 (C
1, Rf = 0.68, UV). IR (neat) νmax/cm−1 3058 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2997 (C
CH), 2997 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2963 (CH), 2869, 2748, 2720, 2684, 1938, 1726 (C
CH), 2963 (CH), 2869, 2748, 2720, 2684, 1938, 1726 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1609 (C
O), 1609 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1496, 1483, 1459, 1409, 1282, 1273, 1219, 1185, 1116, 1089, 1062 δH(500 MHz; CDCl3; Me4Si) 1.34 (9 H, s, tBu CH3 groups), 2.11 (3 H, d, JH,H 6.0, CCH3), 7.30–7.37 [3 H, m, JH,H 6.0, N–CH–O, BimC(5)-H, BimC(6)-H], 7.46 and 7.96 (2 × 2H, AA′ BB′, JAB 8.5, Ph-H), 7.70 [1 H, d, JH,H 8.0, BimC(4)-H or BimC(7)-H], 7.84 [1 H, s, JH,H 7.5, BimC(4)-H or BimC(7)-H], 8.24 [1 H, s, BimC(2)-H]; δC(125 MHz; CDCl3; Me4Si) 20.10 (CH–CH3 group), 31.05 (tBu CH3 groups), 35.18 (tBu C–CH3), 75.43 (N–CH–O), 110.98 [BimC(7)], 120.62 [BimC(4)], 122.86 [BimC(5), BimC(6)], 123.62 [BimC(5), BimC(6)], 125.55 [PhC(3)], 126.00 [PhC(1)], 129.77 [PhC(2)], 132.47 [Bim(8)], 141.13 [Bim(9)], 143.97 [Bim(2)], 157.62 [PhC(4)], 165.08 (COO); MS (ESI): exact mass calcd for C20H22N2O2: 322.17, found m/z 323.200 (M+, 100%). Anal. calcd for C20H22N2O2: C, 74.51; H, 6.88; N, 8.69. Found: C, 74.49; H, 6.90; N, 8.68.
C), 1496, 1483, 1459, 1409, 1282, 1273, 1219, 1185, 1116, 1089, 1062 δH(500 MHz; CDCl3; Me4Si) 1.34 (9 H, s, tBu CH3 groups), 2.11 (3 H, d, JH,H 6.0, CCH3), 7.30–7.37 [3 H, m, JH,H 6.0, N–CH–O, BimC(5)-H, BimC(6)-H], 7.46 and 7.96 (2 × 2H, AA′ BB′, JAB 8.5, Ph-H), 7.70 [1 H, d, JH,H 8.0, BimC(4)-H or BimC(7)-H], 7.84 [1 H, s, JH,H 7.5, BimC(4)-H or BimC(7)-H], 8.24 [1 H, s, BimC(2)-H]; δC(125 MHz; CDCl3; Me4Si) 20.10 (CH–CH3 group), 31.05 (tBu CH3 groups), 35.18 (tBu C–CH3), 75.43 (N–CH–O), 110.98 [BimC(7)], 120.62 [BimC(4)], 122.86 [BimC(5), BimC(6)], 123.62 [BimC(5), BimC(6)], 125.55 [PhC(3)], 126.00 [PhC(1)], 129.77 [PhC(2)], 132.47 [Bim(8)], 141.13 [Bim(9)], 143.97 [Bim(2)], 157.62 [PhC(4)], 165.08 (COO); MS (ESI): exact mass calcd for C20H22N2O2: 322.17, found m/z 323.200 (M+, 100%). Anal. calcd for C20H22N2O2: C, 74.51; H, 6.88; N, 8.69. Found: C, 74.49; H, 6.90; N, 8.68.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (4
ethyl acetate (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as an eluent to give aza-Markovnikov adduct 17 as a pale yellow oil (yields can be seen in Table 3). TLC (SiO2 TLC; hexane
1) mixture as an eluent to give aza-Markovnikov adduct 17 as a pale yellow oil (yields can be seen in Table 3). TLC (SiO2 TLC; hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 4
ethyl acetate = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.41, UV). IR (neat) νmax/cm−1 3122, 2964 (CH), 2906 (CH), 2870 (CH), 2427, 2296, 2097, 1931, 1806, 1719 (C
1, Rf = 0.41, UV). IR (neat) νmax/cm−1 3122, 2964 (CH), 2906 (CH), 2870 (CH), 2427, 2296, 2097, 1931, 1806, 1719 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1609 (C
O), 1609 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1519, 1441, 1398, 1256, 1188, 1113, 1089, 1066, 1041, 1014. δH(500 MHz; CDCl3; Me4Si) 1.34 (9 H, s, tBu CH3 groups), 2.02 (3 H, d, JH,H 6.5, CCH3), 6.31 [1 H, t, JH,H 2.0, PyrC(4)], 7.10 (1 H, q, JH,H 6.0, N–CH–O), 7.45 and 7.98 (2 × 2H, AA′BB′, JAB 8.5, Ph-H), 7.62 [1 H, s, PyrC(3)-H], 7.75 [1 H, d, JH,H 2.5, PyrC(5)-H]; δC(125 MHz; CDCl3; Me4Si) 19.42 (CH–CH3 group), 31.07 (tBu CH3 groups), 35.13 (tBu C–CH3), 79.24 (N–CH–O), 106.15 [PyrC(4)], 125.40 [PhC(3)], 126.46 [PhC(1)], 129.81 [PhC(2)], 129.87 [PyrC(5)], 140.46 [PyrC(3)], 157.30 [PhC(4)], 165.35 (COO); MS (ESI): exact mass calcd for C16H20N2O2: 272.15, found m/z 273.200 (M+, 100%). Anal. calcd for C16H20N2O2: C, 70.56; H, 7.40; N, 10.29. Found: C, 70.49; H, 7.45; N, 10.23.
C), 1519, 1441, 1398, 1256, 1188, 1113, 1089, 1066, 1041, 1014. δH(500 MHz; CDCl3; Me4Si) 1.34 (9 H, s, tBu CH3 groups), 2.02 (3 H, d, JH,H 6.5, CCH3), 6.31 [1 H, t, JH,H 2.0, PyrC(4)], 7.10 (1 H, q, JH,H 6.0, N–CH–O), 7.45 and 7.98 (2 × 2H, AA′BB′, JAB 8.5, Ph-H), 7.62 [1 H, s, PyrC(3)-H], 7.75 [1 H, d, JH,H 2.5, PyrC(5)-H]; δC(125 MHz; CDCl3; Me4Si) 19.42 (CH–CH3 group), 31.07 (tBu CH3 groups), 35.13 (tBu C–CH3), 79.24 (N–CH–O), 106.15 [PyrC(4)], 125.40 [PhC(3)], 126.46 [PhC(1)], 129.81 [PhC(2)], 129.87 [PyrC(5)], 140.46 [PyrC(3)], 157.30 [PhC(4)], 165.35 (COO); MS (ESI): exact mass calcd for C16H20N2O2: 272.15, found m/z 273.200 (M+, 100%). Anal. calcd for C16H20N2O2: C, 70.56; H, 7.40; N, 10.29. Found: C, 70.49; H, 7.45; N, 10.23.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (1
ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as an eluent to give aza-Markovnikov adducts 18 and 19 as pale yellow oils (yields can be seen in Table 3).
1) mixture as an eluent to give aza-Markovnikov adducts 18 and 19 as pale yellow oils (yields can be seen in Table 3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 1
ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.48, UV). IR (neat) νmax/cm−1 3130, 2963 (CH), 2870 (CH), 2389, 2300, 2096, 1938, 1719 (C
1, Rf = 0.48, UV). IR (neat) νmax/cm−1 3130, 2963 (CH), 2870 (CH), 2389, 2300, 2096, 1938, 1719 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1608 (C
O), 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1573, 1460, 1409, 1365, 1339, 1262, 1187, 1078, 1034, 1011. 1H NMR (500 MHz, CDCl3): δ (ppm) 1.34 (9 H, s, tBu CH3 groups), 2.13 (3 H, d, JH,H 6.5, CCH3), 7.32 (1 H, q, JH,H 6.5, N–CH–O), 7.48 and 7.98 (2 × 2 H, AA′BB′, JAB 8.5, Ph-H), 7.74 [1 H, s, TriazC(5)-H], 7.90 [1 H, s, TriazC(4)-H]; δC(125 MHz; CDCl3; Me4Si) 19.69 (CH–CH3 group), 31.04 (tBu CH3 groups), 35.20 (tBu C–CH3), 77.98 (N–CH–O), 125.58 [PhC(3)], 123.63 [TriazC(3)], 126.19 [PhC(1)], 129.89 [PhC(2)], 135.15 [TriazC(4)], 157.86 [PhC(4)], 164.95 (COO); MS (ESI): exact mass calcd for C15H19N3O2: 273.15, found m/z 274.100 (M+, 177.43%). Anal. calcd for C15H19N3O2: C, 65.91; H, 7.01; N, 15.37. Found: C, 65.82; H, 7.18; N, 15.35.
C), 1573, 1460, 1409, 1365, 1339, 1262, 1187, 1078, 1034, 1011. 1H NMR (500 MHz, CDCl3): δ (ppm) 1.34 (9 H, s, tBu CH3 groups), 2.13 (3 H, d, JH,H 6.5, CCH3), 7.32 (1 H, q, JH,H 6.5, N–CH–O), 7.48 and 7.98 (2 × 2 H, AA′BB′, JAB 8.5, Ph-H), 7.74 [1 H, s, TriazC(5)-H], 7.90 [1 H, s, TriazC(4)-H]; δC(125 MHz; CDCl3; Me4Si) 19.69 (CH–CH3 group), 31.04 (tBu CH3 groups), 35.20 (tBu C–CH3), 77.98 (N–CH–O), 125.58 [PhC(3)], 123.63 [TriazC(3)], 126.19 [PhC(1)], 129.89 [PhC(2)], 135.15 [TriazC(4)], 157.86 [PhC(4)], 164.95 (COO); MS (ESI): exact mass calcd for C15H19N3O2: 273.15, found m/z 274.100 (M+, 177.43%). Anal. calcd for C15H19N3O2: C, 65.91; H, 7.01; N, 15.37. Found: C, 65.82; H, 7.18; N, 15.35.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 1
ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rf = 0.82, UV). IR (neat) νmax/cm−1 3428, 2964 (CH), 2869 (CH), 2399, 2281, 2098, 1939, 1724 (C
1, Rf = 0.82, UV). IR (neat) νmax/cm−1 3428, 2964 (CH), 2869 (CH), 2399, 2281, 2098, 1939, 1724 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1609 (C
O), 1609 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1572, 1463, 1410, 1365, 1343, 1267, 1242, 1188, 1089, 1061, 1015. δH(500 MHz; CDCl3; Me4Si) 1.33 (9 H, s, tBu CH3 groups), 2.06 (3 H, d, JH,H 6.5, CCH3), 7.42–7.44 [1 H, m, N–CH–O], 7.44 and 7.98 (2 × 2 H, AA′BB′, JAB 8.5, Ph-H), 7.72 [2 H, s, TriazC(4)-H, TriazC(5)-H]; δC(125 MHz; CDCl3; Me4Si) 19.38 (CH-CH3 group), 31.06 (tBu CH3 groups), 35.14 (tBu C-CH3), 81.46 (N–CH–O), 125.42 [PhC(3)], 126.19 [PhC(1)], 129.90 [PhC(2)], 135.15 [TriazC(4), TriazC(5)], 157.38 [PhC(4)], 164.67 (COO); MS (ESI): exact mass calcd for C15H19N3O2: 273.15, found m/z 274.200 (M+, 91.42%). Anal. calcd for C15H19N3O2: C, 65.91; H, 7.01; N, 15.37. Found: C, 65.79; H, 7.20; N, 15.34.
C), 1572, 1463, 1410, 1365, 1343, 1267, 1242, 1188, 1089, 1061, 1015. δH(500 MHz; CDCl3; Me4Si) 1.33 (9 H, s, tBu CH3 groups), 2.06 (3 H, d, JH,H 6.5, CCH3), 7.42–7.44 [1 H, m, N–CH–O], 7.44 and 7.98 (2 × 2 H, AA′BB′, JAB 8.5, Ph-H), 7.72 [2 H, s, TriazC(4)-H, TriazC(5)-H]; δC(125 MHz; CDCl3; Me4Si) 19.38 (CH-CH3 group), 31.06 (tBu CH3 groups), 35.14 (tBu C-CH3), 81.46 (N–CH–O), 125.42 [PhC(3)], 126.19 [PhC(1)], 129.90 [PhC(2)], 135.15 [TriazC(4), TriazC(5)], 157.38 [PhC(4)], 164.67 (COO); MS (ESI): exact mass calcd for C15H19N3O2: 273.15, found m/z 274.200 (M+, 91.42%). Anal. calcd for C15H19N3O2: C, 65.91; H, 7.01; N, 15.37. Found: C, 65.79; H, 7.20; N, 15.34.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Research, Development and Innovation office (former OTKA, grant number K112289), the Servier-Beregi PhD Research Fellowship and the New Széchenyi Development Plan (TÁMOP_4.2.1/B-09/1/KMR-2010-0002) is gratefully acknowledged.Notes and references
- S. France, D. J. Guerin, S. J. Miller and T. Lectka, Chem. Rev., 2003, 103, 2985 CrossRef CAS PubMed.
- K. Kacprzak and J. Gawronski, Synthesis, 2001, 961 CAS.
- T. Marcelli and H. Hiemstra, Synthesis, 2010, 1229 CrossRef CAS.
- E. M. O. Yeboah, S. O. Yeboah and G. S. Singh, Tetrahedron, 2011, 67, 1725 CrossRef CAS.
- M. North, Sustainable Catalysis: Without Metals or Other Endangered Elements, Part 2, 2016 Search PubMed.
- C. E. Song, Cinchona Alkaloids in Synthesis and Catalysis: Ligands, Immobilization and Organocatalysis, Weinheim, 2009 Search PubMed.
- G. Tanriver, B. Dedeoglu, S. Catak and V. Aviyente, Acc. Chem. Res., 2016, 49, 1250 CrossRef CAS PubMed.
- D. Susam and C. Tanyeli, New J. Chem., 2017, 41, 3555 RSC.
- T. Penaska, K. Ormandyova, M. Meciarova, J. Filo and R. Sebesta, New J. Chem., 2017, 41, 5506 RSC.
- S. Karahan and C. Tanyeli, New J. Chem., 2017, 41, 9192 RSC.
- S. W. Lin, Q. Sun, R. T. Li, T. M. Cheng and Z. M. Ge, Synthesis, 2007, 1933 CAS.
- T. Yano, H. Tomioka, T. Ishiwatari and N. Hirata, Chem. Abstr., 1992, 116 Search PubMed.
- T. Yano; H. Tomioka; T. Ishiwatari and N. Hirata, JP03232805, 1991.
- S. Ozaki, Y. Watanabe, T. Hoshiko, H. Mizuno, K. Ishikawa and H. Mori, Chem. Pharm. Bull., 1984, 32, 733 CrossRef CAS PubMed.
- J. C. Sih, W. B. Im, A. Robert, D. R. Graber and D. P. Blakeman, J. Med. Chem., 1991, 34, 1049 CrossRef CAS PubMed.
- W. B. Wu, N. Wang, J. M. Xu, Q. Wu and X. F. Lin, Chem. Commun., 2005, 2348 RSC.
- W. B. Wu, J. M. Xu, Q. Wu, D. S. Lv and X. F. Lin, Adv. Synth. Catal., 2006, 348, 487 CrossRef CAS.
- X. W. Chen, X. H. Li, H. B. Song, Y. Qian and F. R. Wang, Tetrahedron Lett., 2011, 52, 3588 CrossRef CAS.
- G. Szekely, M. F. Jimenez-Solomon, P. Marchetti, J. F. Kim and A. G. Livingston, Green Chem., 2014, 16, 4440 RSC.
- J. F. Kim, G. Szekely, M. Schaepertoens, I. B. Valtcheva, M. F. Jimenez-Solomon and A. G. Livingston, ACS Sustainable Chem. Eng., 2014, 2, 2371 CrossRef CAS.
- G. Szekely, I. B. Valtcheva, J. F. Kim and A. G. Livingston, React. Funct. Polym., 2015, 86, 215 CrossRef CAS.
- L. Peeva, J. D. Burgal, S. Vartak and A. G. Livingston, J. Catal., 2013, 306, 190 CrossRef CAS.
- W. E. Siew, C. Ates, A. Merschaert and A. G. Livingston, Green Chem., 2013, 15, 663 RSC.
- R. A. Sheldon, Green Chem., 2007, 9, 1273 RSC.
- R. A. Sheldon, Chem. Commun., 2008, 3352 RSC.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879 RSC.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546 RSC.
- T. Welton, Proc. R. Soc. London, Ser. A, 2015, 471, 1 CrossRef PubMed.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- M. Kidwai, N. K. Mishra and A. Jahan, Chin. Chem. Lett., 2011, 22, 417 CrossRef CAS.
- H. Y. Bae, S. Some, J. H. Lee, J. Y. Kim, M. J. Song, S. Lee, Y. J. Zhang and C. E. Song, Adv. Synth. Catal., 2011, 353, 3196 CrossRef CAS.
- A. Moran, A. Hamilton, C. Bo and P. Melchiorre, J. Am. Chem. Soc., 2013, 135, 9091 CrossRef CAS PubMed.
- P. Melchiorre, M. Marigo, A. Carlone and G. Bartoli, Angew. Chem., Int. Ed., 2008, 47, 6138 CrossRef CAS PubMed.
- J. F. Kim, G. Szekely, I. B. Valtcheva and A. G. Livingston, Green Chem., 2014, 16, 133 RSC.
- C. Didaskalou, S. Buyuktiryaki, R. Kecili, C. P. Fonte and G. Szekely, Green Chem., 2017, 19, 3116 RSC.
- M. Schaepertoens, C. Didaskalou, J. F. Kim, A. G. Livingston and G. Szekely, J. Membr. Sci., 2016, 514, 646 CrossRef CAS.
- M. Razali, C. Didaskalou, J. F. Kim, M. Babaei, E. Drioli, Y. M. Lee and G. Szekely, ACS Appl. Mater. Interfaces, 2017, 9, 11279 CAS.
- M. Kidwai, M. Lal, N. K. Mishra and A. Jahan, Green Chem. Lett. Rev., 2013, 6, 63 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: R, NMR and MS data for compounds 13–19. See DOI: 10.1039/c8nj01277f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |






