Factors affecting charge transfer in tetraiodide dianions
Abstract
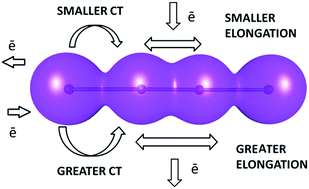
Thirty-one examples of crystal structures containing discrete tetraiodide I42− dianions were identified from the Cambridge Structural Database (CSD) and analyzed in detail in order to find the factors influencing the geometry of this rare fragment. The central I–I bond is always significantly shorter than the terminal I–I contacts; however, no significant correlation between the lengths of these two different kinds of bonds was found. Instead, it was observed that the intermolecular interactions are at least partially responsible for the changes in the geometry of the dianion. It has been found that, in the structures in which cations interact with hydrogen bonds with terminal iodine atoms of I42−, the elongation of the central I–I bond is systematically smaller than in the structures without such interactions. Other intermolecular interactions can also play a significant, albeit secondary role, in the processes influencing the geometry of the anion. These effects were analyzed by means of topological analysis of the electron density in the vicinity of I42− anions (critical point analysis) and of the Hirshfeld surfaces. An explanation of the observed tendencies has been proposed.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...