May halogen bonding interactions compete with Cu⋯Cl semi-coordinate bonds? Structural, magnetic and theoretical studies of two polymorphs of trans-bis(5-bromo-2-chloro pyridine)dichlorocopper(ii) and trans-bis(2,5-dichloropyridine)dichlorocopper(ii)†
Abstract
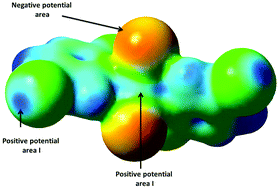
Two polymorphs of Cu(25dcp)2Cl2 [Cu(25dcp)2Cl2_p1 and Cu(25dcp)2Cl2_p2] and Cu(5b2cp)2Cl2 [Cu(5b2cp)2Cl2_p1 and Cu(5b2cp)2Cl2_p2] were prepared and characterized by single crystal X-ray diffraction (25dcp = 2,5-dichloropyridine and 5b2cp = 5-bromo-2-chloropyridine). The four structures crystallize in the monoclinic crystal system: three of them (Cu(25dcp)2Cl2_p1, Cu(5b2cp)2Cl2_p2 and Cu(25dcp)2Cl2_p2) crystallize in the C2/c space group and the fourth one (Cu(5b2cp)2Cl2_p1) in the C2/m space group. Cu(5b2cp)2Cl2_p1 and Cu(25dcp)2Cl2_p1 are structurally isomorphous. The formation of two polymorphs for each complex is a result of competition between Cl⋯Cu semi-coordinate bonds and C–X⋯Cl–Cu (X = Cl or Br) halogen bonding interactions. The formation of C–X⋯Cl–Cu halogen bonding alone resulted in crystallization of Cu(25dcp)2Cl2_p1 and Cu(5b2cp)2Cl2_p1, whereas the formation of the Cu⋯Cl semi-coordinate bonds resulted in the formation of Cu(25dcp)2Cl2_p2 and Cu(5b2cp)2Cl2_p2. To our knowledge, these are the first examples in which the competition between the halogen bonding interactions and the semi-coordinate Cu⋯Cl resulted in the formation of different polymorphs. The calculated electrostatic potential was used to rationalize the formation of the different polymorphs. The magnetic properties of Cu(5b2cp)2Cl2_p1 were studied; it is found to obey an antiferromagnetic chain model. This magnetic behavior was rationalized using the two-halide exchange pathway. Structurally, Cu(5b2cp)2Cl2_p1 forms a chain structure based on Cu–Cl⋯Cl–Cu interactions. The mapped electron density with spin density showed the presence of a spin-density-end-cap along the Cu–Cl bond. This spin-density-end-cap corroborates the observed antiferromagnetic interactions.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...