Influence of water on the structure and magnetic properties of a copper bromide coordination compound with 3-amino-4-ethoxycarbonylpyrazole†
Abstract
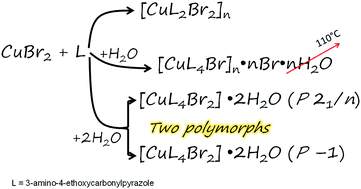
The influence of water on a polynuclear [CuL2Br2]n (1) copper bromide compound with 3-amino-4-ethoxycarbonylpyrazole (L) is investigated. The abundance of water in solution results in the formation of three new complexes with drastically different coordination units: polynuclear {[CuL4Br]Br}n·nH2O (2) and two mononuclear polymorphs [CuL4Br2]·2H2O (monoclinic – 3, triclinic – 4). The crystal structures are determined for all three complexes. Complex 2 has a weak (≪1 cm−1) superexchange interaction between copper ions and bromide ions. Complexes 3 and 4 have no exchange interaction. It is found that the two polynuclear complexes may transform into each other through dissolution followed by recrystallization under certain conditions.


 Please wait while we load your content...
Please wait while we load your content...