Arsenic trioxide preferentially binds to the ring finger protein PML: understanding target selection of the drug†
Abstract
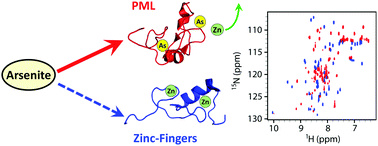
Arsenic trioxide (ATO) is used in the clinic for the treatment of acute promyelocytic leukemia by targeting the protein PML. However, many zinc-finger proteins could also be reactive to arsenic in cells. Here we found that ATO preferentially binds to the ring-finger domain of PML in a protein mixture with zinc-finger domains. These results provide the molecular basis of the target selection of ATO in cells.


 Please wait while we load your content...
Please wait while we load your content...