Synthesis and biological evaluation of new 3-amino-2-azetidinone derivatives as anti-colorectal cancer agents†
Abstract
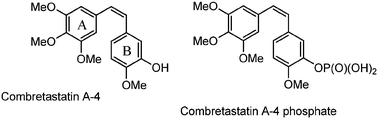
Several synthetic combretastatin A4 (CA-4) derivatives were recently prepared to increase the drug efficacy and stability of the natural product isolated from the South African tree Combretum caffrum. A group of ten 3-amino-2-azetidinone derivatives, as combretastatin A4 analogues, was selected through docking experiments, synthesized and tested for their anti-proliferative activity against the colon cancer SW48 cell line. These molecules, through the formation of amide bonds in position 3, allow the synthesis of various derivatives that can modulate the activity with great resistance to hydrolytic conditions. The cyclization to obtain the 3-aminoazetidinone ring is highly diastereoselective and provides a trans biologically active isomer under mild reaction conditions with better yields than the 3-hydroxy-2-azetidinone synthesis. All compounds showed IC50 values ranging between 14.0 and 564.2 nM, and the most active compound showed inhibitory activity against tubulin polymerization in vitro, being a potential therapeutic agent against colon cancer.


 Please wait while we load your content...
Please wait while we load your content...