Plasma processes to detect fluorine with ICPMS/MS as [M–F]+: an argument for building a negative mode ICPMS/MS†
Nor Laili
Azua Jamari
,
Arne
Behrens
,
Andrea
Raab
 ,
Eva M.
Krupp
and
Jörg
Feldmann
,
Eva M.
Krupp
and
Jörg
Feldmann
 *
*
Trace Element Speciation Laboratory (TESLA), Department of Chemistry, University of Aberdeen, Aberdeen, AB24 3UE, UK. E-mail: j.feldmann@abdn.ac.uk
First published on 16th May 2018
Abstract
Detection of fluorine with commercial ICPMS is impossible due to the high ionisation potential (IP) of fluorine. A novel approach through the formation of fluorine-containing polyatomic ions [M–F]+ in the plasma allows the successful detection of F at sub ppm levels by ICPMS/MS. Two theories behind [M–F]+ formation have been proposed, yet there is no clear understanding about the mechanism. Here, different metal solutions were tested for the characterisation of plasma processes in the formation of [M–F]+. Three characteristics: high [M–F]+ bond dissociation energy (BDE), low [M–O]+ BDE and low IP were found to be essential to get the highest sensitivity for [M–F]+. It was found that for elements with a higher [M–F]+ BDE than [M–O]+ BDE, the sensitivity decreases linearly with the second IP of the element, meaning that the major process in the plasma is the harvesting of F− by M2+ to form [M–F]+. Barium exhibited the highest sensitivity for [M–F]+. However, the robustness of this approach was questioned due to matrix effects, hence an argument for re-developing negative ion ICPMS/MS was discussed in which detection limits in the sub-ppb range could be reached.
Introduction
Inductively coupled plasma mass spectrometry (ICPMS) is well known as a powerful analytical tool for analysing almost all elements in the periodic table with lower detection limits. Since the introduction of ICPMS in the 1980s and until now this technique has been mainly used in the positive ion mode. As the ionisation potential (IP) of fluorine is high (17 eV), no significant amounts of F+ are generated in the plasma. Therefore, fluorine cannot be sensitively detected directly using commercial argon plasma ICPMS. Due to significant interference from polyatomic ions formed in ICPMS, an attempt has been made to use this concept to detect fluorine by producing [M–F]+ ions and separate them from other polyatomic ions using ICPMS/MS.Polyatomic ions form from the reaction and collision between ions and/or atoms arising from elements, which are present abundantly in the plasma, sample and/or solvent. The temperature is a key factor in determining at which point in the plasma the polyatomic ions are formed. This is concurrently related to the bond dissociation energy (BDE) of the polyatomic ions as ions with low BDE can be broken at high temperature while ions with high BDE stay stable in the plasma. Hence, a metal that has high BDE with fluorine has been chosen by Yamada to form poly-nuclear fluorine ions in the plasma.1
An attempt has been made to use a barium solution to form significant amounts of [BaF]+ so that detection limits around 0.05 mg L−1 could be achieved when oxygen or ammonia was used as the reaction gas in ICPMS/MS.2–4 This approach was then applied for total fluorine analysis in drinking water and food as well as fluorine speciation analysis.2–5 Two theories exist on how these poly-nuclear ions are generated in an argon plasma. However, the mechanism behind these theories remains unclear.
| 138Ba+ + 19F0 ⇌ [138Ba19F+] | (1) |
| 138Ba2+ + 19F− → [138Ba19F]+ | (2) |
In the past, few attempts have been made to detect fluorine with negative-ion ICPMS.6,7 Apparently, a detection limit of 0.4 mg L−1 for fluorine can be achieved in negative ion mode with a simple quadrupole instrument capable of switching from positive to negative ion detection as is common for a molecular mass spectrometer.6 Despite the lack of collision or reaction cells and the resulting high background, better limits of detection for fluorine and chlorine were observed using the negative mode detection than the positive mode. Hence, with today's technology of triple quadrupoles and lens configuration, lower background noise coming from polyatomic interference ions, for example [16O1H3]− or [18O1H]− can be eliminated and better extraction of analyte ions would be expected in negative mode ICPMS/MS. However, these ICPMS instruments are no longer commercially available nowadays, despite their high potential in analysing halogens as well as other negative ions. Therefore, in this study, to gain a deeper understanding about the mechanism of [M–F]+ formation, characterisation of the plasma processes in the formation of [M–F]+ was carried out. In addition, the implication to improve the detection limits and robustness of fluorine detection using ICPMS/MS was also studied.
Experimental section
Chemicals, standards and reagents
Milli-Q water (18 MΩ cm, Smart2Pure, Thermo Fisher Scientific, UK) was used for all analytical purposes. Fluorine standards for optimisation and calibration were prepared from potassium fluoride (Fisher Scientific, UK), sodium fluoride (Fisher Scientific, UK) and hydrogen fluoride (Fisher Scientific, UK). Metal standards were prepared from Ba(NO3)2 (BDH, UK), Ca (SCP science, Canada), Ce (Sigma-Aldrich, Switzerland), Er (Inorganic Ventures, USA), Eu (SCP science, Canada), Gd (SCP science, Canada), La (Sigma-Aldrich, Switzerland), Sr(NO3)2 (BDH, UK), Pr (Sigma-Aldrich, Switzerland) and Yb (SCP science, Canada).Instrumental setup
Optimisation and analysis were carried out using an 8800 Triple Quadrupole ICPMS/MS instrument (Agilent Technologies, UK) with a micromist nebuliser, nickel sampler and skimmer cone and S-lens. As shown in Fig. 1, the instrumental set-up for polyatomic ion [M–F]+ analysis followed a previous study.4 The ICPMS/MS parameters described in Jamari et al.4 were optimised for each metal (M) to get the maximum sensitivity, while for [Ba–F]+, the same parameters as in our previous study were used. The ICPMS/MS was tuned daily for maximum sensitivity and Table 1 shows the details of the investigated [M–F]+ while Table S1† shows the optimum parameters for each metal investigated. The sensitivity of each [M–F]+ was illustrated as the corrected SBR, which was calculated based on the isotopic abundance of the measured isotopes for each metal and corrected for moles (calculations shown in the ESI†).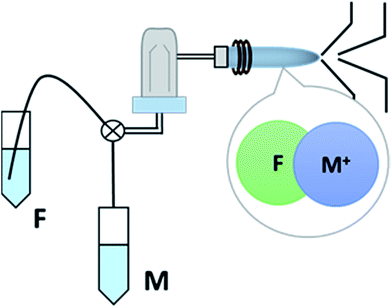 | ||
| Fig. 1 Instrumental set-up for fluorine detection using ICPMS/MS. M is the metal containing solution, while F is the fluorine containing solution. | ||
| Metal | Ba, Sr, Yb, Ca, Eu, Er, Gd, La, Pr |
| m/z | 157 [Ba–F]+, 107 [Sr–F]+, 193 [Yb–F]+, 59 [Ca–F]+, 172 [Eu–F]+, 185 [Er–F]+, 179 [Gd–F]+, 158 [La–F]+, 160 [Pr–F]+, 159 [Ce–F]+ |
| Metal concentration | 50 mg L−1 |
| F concentration | 0.1–10 mg L−1 |
Results and discussion
Interference is the most common problem in ICPMS analysis involving atoms from the plasma, solvent and entrained air. Like other ICPMS analyses, metal-fluorides also suffer from isobaric interference that have the same mass-to-charge (m/z) as the analyte. For example, at m/z 157, [Ba–F]+ have isobaric interference from barium oxide ions (e.g., [138Ba18O1H]+, [137Ba18O2H]+, [138Ba16O1H3]+, and [138Ba17O2H]+). As our previous study revealed, plasma conditions are crucial for the formation of polyatomic ions and reducing the interferences.4Two mechanisms shown in eqn (1) and (2) were proposed for the formation of [Ba–F]+. Since Ba has a low first IP (5.2 eV), the mechanism in eqn (1) was expected to be the dominant process occurring in the plasma. However, the formation of polyatomic ions [Ba–F]+ was more efficient in hot plasma compared to cool plasma (Fig. 2a). This hot environment could promote the formation of the doubly charged ions, Ba2+, since Ba has a low second IP (10.0 eV). Apart from this, the highest intensity of [Ba–F]+ was mainly deep in the plasma at a low sampling position (7.5–8.5 mm). The maximum signal-to-background ratio (SBR) of [Ba–F]+ also did not coincide with the maximum intensities of either Ba+ or Ba2+ (Fig. 2b). The location in the plasma for the highest intensity of [Ba–F]+ did not coincide with the location where barium showed the highest intensities for both Ba+ and Ba2+ respectively. This means that the formation [Ba–F]+ is limited by F (either F0 or F−) rather than the amount and type of barium ion in the plasma.
In order to identify whether the amount of barium would change the location of the highest intensity of [Ba–F]+, the amount of added Ba was varied together with the sampling location. As shown in Fig. 3, the place of the highest sensitivity of [Ba–F]+ was dependent on the Ba concentration. The sensitivity of [Ba–F]+ increases with an increase of the added Ba concentrations between 10 and 100 mg L−1. However, the counts barely increase from 50 to 100 mg L−1. Hence it is reasonable to use 50 mg L−1 to avoid clogging of the sampler and skimmer cone with too high concentrations of barium (Fig. S2†). When there is excess metal present in the plasma, [M–F]+ can be detected. Since Ba is present abundantly in the plasma, this suggests that instead of Ba species, F plays a role in limiting the formation of [Ba–F]+ polyatomic ions. Due to the fact that F cannot be measured directly by ICPMS/MS, the influence of thermochemical properties was studied to gain better understanding about which mechanism involved in the formation of [BaF]+ polyatomic ions is the predominant one.
 | ||
| Fig. 3 The normalized sensitivity of [Ba–F]+ at different Ba concentrations recorded at different sampling positions. | ||
As has been described by Yamada,1 thermochemical properties of metals such as BDE and IP are the key factors for the efficient formation of the [M–F]+ polyatomic ions. Since oxygen is present abundantly in the plasma, the BDE between fluorine and oxygen-containing polyatomic ions needs to be considered as well. Ba with a high affinity to F (a high BDE for [Ba–F]+ of 6.39 eV), a low affinity to O (a low BDE for [Ba–O]+ of 4.00 eV) and a low first and second IP was expected to be the best candidate for efficient [M–F]+ formation. Due to the given working hypothesis that the stability of [M–F]+ is essential to achieve the highest sensitivity for a fluorine containing polyatomic ion, a series of metal solutions were used to investigate this hypothesis. Table 2 and Fig. S1† illustrate the BDE and IP of different metals (M) from alkaline earth to lanthanide metals.
| Metal | 1st IP (eV) | 2nd IP (eV) | BDE [M–F]+ (eV) | BDE [M–O]+ (eV) | References |
|---|---|---|---|---|---|
| Be | 9.32 | 18.2 | 6.21 | 4.05 | 8–10 |
| Mg | 7.65 | 15.0 | 4.26 | 2.26 | |
| Ca | 6.11 | 11.9 | 5.42 | 3.12 | |
| Sr | 5.70 | 11.0 | 5.43 | 3.06 | |
| Ba | 5.21 | 10.0 | 6.39 | 4.00 | 11 and 12 |
| La | 5.58 | 11.1 | 6.83 | 8.73 | |
| Ce | 5.54 | 10.8 | 6.35 | 8.80 | |
| Pr | 5.46 | 10.5 | 6.34 | 8.23 | |
| Nd | 5.53 | 10.7 | 6.24 ± 0.21 | 7.76 | |
| Sm | 5.64 | 11.7 | 6.29 ± 0.16 | 5.80 | |
| Eu | 5.67 | 11.2 | 6.05 ± 0.16 | 4.00 | |
| Gd | 6.15 | 12.1 | 6.10 ± 0.25 | 7.47 | |
| Tb | 5.86 | 11.5 | 6.43 | 7.33 | |
| Dy | 5.94 | 11.7 | 5.54 ± 0.25 | 6.11 | |
| Ho | 6.02 | 11.8 | 5.33 ± 0.21 | 6.24 | |
| Er | 6.10 | 11.9 | 5.69 ± 0.25 | 5.96 | |
| Tm | 6.20 | 12.0 | 5.57 ± 0.16 | 4.92 | |
| Yb | 6.25 | 12.2 | 5.78 ± 0.15 | 3.87 | |
| Lu | 5.43 | 13.9 | 3.91 | 5.34 |
Fig. 4a displays the sensitivity of each [M–F]+versus the BDE of metal-fluoride ions. All metals have similar BDEs with fluorine, but only Ba, Sr and Eu exhibited a significant SBR as [M–F]+, whereas very low or negative SBRs were recorded by other metals. These data demonstrate that a strong [M–F]+ bond alone was not sufficient to explain the formation and detection of [M–F]+. As the SBR is highly dependent on the ratio between the [M–F]+ and the [M–OH]+ species in the plasma, it is necessary to observe the influence of the [M–O]+ bond as well. Fig. 4b shows the SBRs of various [M–F]+versus the differences of BDEs between [M–F]+ and [M–O]+. The metals with a higher BDE for [M–O]+ than the [M–F]+ bond are illustrated as red triangles resulting in large amounts of interfering metal oxide ions in the plasma; hence the low SBR. Yet, this does not explain the low sensitivity of Yb and Ca. Although both have higher BDE for [M–F]+ than [M–O]+, they exhibited lower sensitivity compared to Ba, Sr and Eu. Hence, the BDE differences between the fluorine and the oxide ions would not explain the formation and detection of the [M–F]+ solely.
To find an explanation why Ba, Sr and Eu showed significant SBRs although their [M–F]+ bond is comparable with others; another thermodynamic parameter was studied. The first and the second IPs of each metal were investigated to explain the sensitivity of [M–F]+. Based on Fig. 5a and b, metals with good sensitivity (Ba, Sr and Eu) have lower first and second IPs compared to Ca and Yb, although all of them have positive BDE differences between [M–F]+ and [M–O]+. Meanwhile, metals with negative BDE differences (illustrated as red triangles) have low SBR despite their lower first and second IPs; especially Ce, Pr and La. A correlation study also reveals that there is a significant relationship between sensitivity and low IPs (p-value < 0.05) (Table 3). This result discloses that both BDE and IP are essential for the formation of [M–F]+ and as shown in Fig. 5b, Ba with the lowest IPs exhibit the highest sensitivity. Hence, high affinity of Ba with fluorine, low affinity of Ba with oxygen and low IPs make Ba the most promising metal for the efficient formation of [M–F]+ with the highest sensitivity which is in accordance with the working hypothesis.
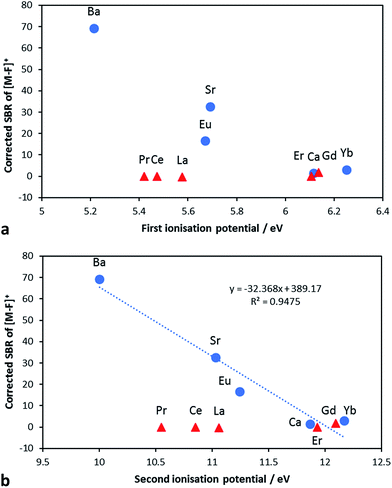 | ||
| Fig. 5 (a) Corrected SBR of [M–F]+ dependent on the first IP of metals (M to M+). The symbol description is the same as displayed in Fig. 4b. (b) Optimised SBR of [M–F]+ dependent on the second IP of metals (M+ to M2+). The symbol description is the same as displayed in Fig. 4b. | ||
| SBR of [M–F]+ with first IPs of metals | SBR of [M–F]+ with second IPs of metals | |
|---|---|---|
| Pearson correlation | −0.937 | −0.973 |
| P-Value | 0.019 | 0.005 |
| R 2 | 0.88 | 0.95 |
The elemental behaviour of [M–F]+ formation and detection in relation to the first and the second IPs suggest that the second IP is more important than the first IP for the formation of [M–F]+. This is well illustrated by Eu and Sr. Although Eu has a marginally lower first IP (5.67 eV) than Sr (5.70 eV), the sensitivity of Eu is lower. The stronger linear increase in sensitivity for [M–F]+ with a decreasing second IP illustrates well (Table 3) that the formation of [M–F]+ depends on the occurrence of M2+ rather than M+. As shown in Fig. S3a and b,† similar results were exhibited when the SBR was adjusted based on the molarity of the measured isotope. These findings indicate that the predominant process occurring in the plasma is the binding of M2+ with F− to form [M–F]+.
From our experience, it has been noticeable that the formation of [M–F]+ is notoriously unstable (Fig. S4†) and highly matrix dependent when easy to ionise metals were added to the plasma (Fig. S5 and S6†). No major effect was observed in the presence of methanol, chloride and sulphate indicating that the carbon enhancement effect is not influencing the formation and detection of [Ba–F]+ (Fig. S7†). However, as shown in Fig. 6 by the calibration graph, fluoride solutions containing easily ionisable elements show signal enhancement of [Ba–F]+. This can be explained by the low first IP of potassium (4.3 eV) and sodium (5.1 eV). Both elements, added in excess relative to the F concentration, increase the electron density and therefore may increase the plasma temperature.13 Fluorine has high electron affinity and the excess of electrons might force the equilibrium towards F− (eqn (3)). The higher plasma temperature may also influence the [M–F]+ formation. Since potassium has a lower first IP compared to sodium, higher electron density was expected to be produced in the plasma from the fluoride solution containing potassium. This was however not the case indicating that another process in the plasma is significant for the [Ba–F]+ formation and detection. The higher electron density might also force the equilibrium of Ba2+ towards Ba+, thus reducing the sensitivity of [Ba–F]+ (eqn (4)).
| F0 + e− ⇌ F− | (3) |
| Ba2+ + e− ⇌ Ba+ | (4) |
In comparison with Sr and Eu, the calibration graphs with fluoride solution containing potassium and sodium did not really affect the sensitivity of [M–F]+ (Fig. S6 and S7†). This can be explained by the higher second IP of both Sr and Eu compared to that of Ba, restraining the production of M2+ in the presence of high electron density. Based on these findings, it can be concluded that the predominant process occurring in the plasma is the binding of M2+ with F− to form [M–F]+.
Since F− abundance is most important, it would be useful to sample directly this ion and detect F− directly by the use of a negative mode ICPMS. This has been done in the past with ICPMS designed in the late 1980s by the Hieftje group.6,14
In order to achieve low detection limits for fluorine and make its detection less matrix dependent, F− should be directly detected. This may be achieved by two hypotheses:
(a) Transferring F− into the reaction cell of the ICPMS/MS and convert the negative ion into a positive one [X–F]+ or
(b) Detecting F− in a negative ion mode ICPMS/MS.
Even though option (a) would be possible with any current commercial ICPMS/MS, it is very unlikely to be achieved. This is because it is difficult to find any reactive gases capable of forming doubly charge ions, which react with F− to form a polyatomic positive ion. Hence, it would be more likely to rebuild an ICPMS/MS with negative mode detection to achieve a more robust detection with limits of detection in the lower ppb or even the sub-ppb range.
With today's technology of ICPMS in ion transmission and interference removal, the negative ion ICPMS/MS has high potential in detecting not only halogens but also other negative ions such as nitrogen. Data from Vickers et al.6 have been used to estimate the detection limit of fluorine by using the chlorine value as reference. In 1988, it was reported that chlorine could be detected with three times better sensitivity in negative mode compared to positive mode. By considering the detection limit of chlorine with today's ICPMS/MS sensitivity (4.0 μg L−1),15,16 it is expected that the detection limits of chlorine and fluorine in the lower to the sub ppb range could be achieved in negative mode ICPMS/MS. Furthermore, interferents [18O1H]− which impaired the fluorine detection in negative ion mode of the old ICPMS can now be reduced using a reaction gas in the collision/reaction cell.
ICPMS/MS in negative mode has great potential for halogen detection which simultaneously could solve many problems in environmental and food analysis. Although, ion chromatography and ion selective electrodes are the common analytical tools used for fluoride analysis, it is impossible with these methods to detect organofluorines such as per- and polyfluorinated compounds. Since only targeted methods such as ESI-MS/MS and limited standards are available, many of the organofluorine compounds remain undetected which not only occur in fluorinated polymer compounds but also in pharmaceutical applications. Nowadays, almost 20% of all new pharmaceutical products are fluorinated.17 The metabolism of these compounds cannot easily be studied due to mass balance problems when only molecular mass spectrometry is used. The same issue is further extended to chlorinated compounds and again, mass balance approaches for the biodegradation of chlorinated pesticides or insecticides or the metabolism of chlorinated pharmaceuticals can only be studied when an element-specific detector such as ICPMS is used for non-targeted analysis of body fluids or soil/plant extracts. Therefore, building a new negative-ion ICPMS/MS would not only benefit the organofluorine polymer industry and the pharmaceutical sector but would also serve other communities such as scientists interested in environmental monitoring of fluorinated and chlorinated compounds.
Conclusion
In this work, the formation of fluorine-containing polyatomic ions with different metals using ICPMS/MS was demonstrated. The high BDE of [M–F]+, and relatively low BDE of [M–O]+ together with a low second IP of the metal are the main features required by the metal to form [M–F]+ efficiently in an argon plasma. Ba showed the highest sensitivity for the most promising metal forming fluorine-containing polyatomic ions. The study revealed that harvesting of F− by M2+ in the argon plasma to form [M–F]+ was the predominant process. Although this approach made it possible to detect fluorine at sub-ppm concentration levels with ICPMS/MS, polyatomic ion formation is unstable and matrix dependent. A negative ion ICPMS/MS would be able to push the limits of detection to sub-ppb levels and with potentially less matrix effects. Negative ion ICPMS/MS would not only be useful to detect halogens with low detection limits but also as an element-specific detector for identifying unknown halogen compounds from the environment, food and/or pharmaceutical area.Conflicts of interest
There are no conflicts to declare.Acknowledgements
NLAJ thanks the Malaysian Government (Grant number: RG12824-10) and National Defence University of Malaysia for financial support throughout the study period, while AB thanks the Erasmus programme of the EU. Special thanks to Swedish Research Council for additional financial support (Grant number: FORMAS 1397306) and also to Samira Al Hinai and Amanda Victor for helping in this project. The authors thank Dr Christoph-Cornelius Brombach for the artwork.References
- N. Yamada, in Agilent 8800 ICP-QQQ Application Handbook, Agilent Technologies, 2nd edn, 2015, pp. 38–39 Search PubMed.
- W. Guo, L. Jin, S. Hu and Q. Guo, J. Agric. Food Chem., 2017, 65, 3406–3412 CrossRef PubMed.
- Y. Z. Hu, K. N. Akano and Y. S. Hikamori, Anal. Sci., 2017, 33, 1279 CrossRef PubMed.
- N. L. A. Jamari, J. F. Dohmann, A. Raab, E. M. Krupp and J. Feldmann, J. Anal. At. Spectrom., 2017, 32, 942–950 RSC.
- N. L. A. Jamari, J. F. Dohmann, A. Raab, E. M. Krupp and J. Feldmann, J. Anal. At. Spectrom., 2017, 1–14 Search PubMed.
- G. H. Vickers, D. A. Wilson and G. M. Hieftje, Anal. Chem., 1988, 60, 1808–1812 CrossRef.
- M. Chtaib and J.-P. Schmit, J. Anal. At. Spectrom., 1988, 3, 315–318 RSC.
- S. R. Langhoff, C. W. Bauschlicher and H. Partridge, J. Chem. Phys., 1986, 84, 1687–1695 CrossRef.
- K. Schofield, Chem. Rev., 1967, 67, 707–715 CrossRef.
- H. Partridge, S. R. Langhoff and C. W. Bauschlicher, J. Chem. Phys., 1986, 84, 4489–4496 CrossRef.
- R. J. Ackermann, E. G. Rauh and R. J. Thorn, J. Chem. Phys., 1976, 65, 1027 CrossRef.
- L. A. Kaledin, M. C. Heaven and R. W. Field, J. Mol. Spectrosc., 1999, 193, 285–292 CrossRef PubMed.
- J. A. Olivares and R. S. Houk, Anal. Chem., 1986, 58, 20–25 CrossRef.
- D. A. Wilson, G. H. Vickers, G. M. Hieftje and A. T. Zander, Spectrochim. Acta, Part B, 1987, 42, 29–38 CrossRef.
- N. Sugiyama, Trace Level Analysis of Sulfur, Phosphorus, Silicon and Chlorine in NMP Using the Agilent 8800 Triple Quadrupole ICP-MS, Japan, Tokyo, 2013 Search PubMed.
- B. Divjak, M. Novič and W. Goessler, J. Chromatogr. A, 1999, 862, 39–47 CrossRef PubMed.
- A. Harsanyi and G. Sandford, Green Chem., 2015, 17, 2081–2086 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ja00050f |
| This journal is © The Royal Society of Chemistry 2018 |



