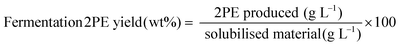Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Production of fermentable species by microwave-assisted hydrothermal treatment of biomass carbohydrates: reactivity and fermentability assessments†
Javier
Remón
 a,
Fabio
Santomauro
b,
Christopher J.
Chuck
a,
Fabio
Santomauro
b,
Christopher J.
Chuck
 *b,
Avtar S.
Matharu
*b,
Avtar S.
Matharu
 a and
James H.
Clark
a and
James H.
Clark
 *a
*a
aGreen Chemistry Centre of Excellence, University of York, Department of Chemistry, Heslington, York, YO10 5DD, UK. E-mail: james.clark@york.ac.uk
bDepartment of Chemical Engineering, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: c.chuck@bath.ac.uk
First published on 6th September 2018
Abstract
This work addresses and compares the production of fermentable species by microwave-assisted hydrothermal treatment of cellulose and hemicellulose (from lignocellulose) and alginic acid (from macroalgae). A reliable reactivity comparison was established at different temperatures (160–210 °C), reaction times (0 and 5 min) and solid/water mass ratios (1/20 and 1/10 g/g). The nature of the carbohydrates and the hydrothermal conditions had a significant influence on the reactivity, which increased as follows: cellulose < hemicellulose < alginic acid. The operating conditions did not influence the global conversion obtained during hydrothermal treatment of cellulose. Conversely, the temperature and reaction time played an important role when processing hemicellulose or alginic acid. In these two cases, increasing the temperature and/or reaction time increased the overall conversion and liquid and gas yields. The liquid hydrolysates were made up of a mixture of oligo-(DP 3–6 and DP > 6) and mono-/di-saccharides, carboxylic acids, ketones and furans. While the chemical composition of the hydrolysates produced from hemicellulose was not affected by the microwave operating conditions, the liquids having a high concentration of DP > 6 oligosaccharides in all cases, the microwave conditions substantially influenced the composition of the liquids produced from cellulose and alginic acid. The former contained high proportions of oligosacharides and saccharides and the latter comprised water soluble DP > 6 oligomers/oligosaccharides, saccharides, carboxylic acids and furans. The yeast Metschnikowia pulcherrima, previously demonstrated to be inhibitor tolerant and to metabolise a range of oligosacchaides, was used to assess the fermentability of the liquid fraction. All the hydrolysates produced were fermentable; their efficiency (standarised yeast biomass growth) decreasing as follows: cellulose (high/low saccharides/inhibitors proportion) > hemicellulose (high/low oligosaccharides/inhibitors proportion) > alginic acid (low/high saccharides/inhibitors proportion). Therefore, the promising results obtained in this work and the intrinsic green nature of the process make this method a very promising route for biomass valorisation, which can help to enable the development of new thermochemical and biological linked routes.
1. Introduction
The development of alternative technologies and more sustainable strategies has become a very important issue to satisfy the energy, chemicals and material consumption requirements of the present petroleum-based society and the well-being of future generations.1,2 In this regard, the use of biomass to produce fuels and chemicals is gaining increasing attention;3 one of the most suitable routes for biomass valorisation being the exploitation of its carbohydrate content. Among the different processes to produce sugars from biomass, microwave-assisted hydrothermal depolymerisation is seen as a promising alternative as it allows the conversion of the carbohydrate content of biomass into fermentable sugar-rich aqueous solutions.4 In addition, sugar degradation takes place to a lesser extent than when acid- or alkali-hydrolysis are used5–8 and microwave-assisted hydrothermal depolymerisation is much faster than acid or enzymatic routes.5,9 The process is flexible and can be used effectively for the pre-treatment and/or upgrading of different biomasses, including both second and third generations of biomass.5–8 The former comprises non-edible lignocellulosic materials and waste residues, largely consisting of cellulose, hemicellulose and lignin;10 while the latter involves micro- and macroalgal matter, which is made up of active compounds such as proteins, nucleic acids and lipids and carbohydrates such as cellulose, hemicellulose and alginic acid.11,12For the development and commercialisation of this route, it is of paramount importance to achieve an optimum control of the process in order to strike a good compromise between sugar production and sugar degradation, as the latter leads to the formation of fermentation inhibitors such as aldehydes, carboxylic acids, ketones and furans.5–8 However, to the best of the authors’ knowledge, little research has been conducted addressing the hydrothermal treatment of the most abundant carbohydrates; i.e. cellulose, hemicellulose and alginic acid, especially comparing their reactivity when treated under the same conditions. In addition, owing to the diverse biomass composition, a reliable analysis of biomass carbohydrates is very important to understand the behaviour of real biomass.
Rogalinski et al.13 studied cellulose decomposition under standard hydrothermal conditions analysing the effect of the temperature. 100% cellulose conversion was achieved within 2 minutes in a batch reactor at 280 °C. They found that rapid heating is crucial to avoid secondary degradation reactions. Sasaki et al.14 analysed the relationship between the cellulose hydrolysis rate and glucose decomposition and concluded that below 350 °C the cellulose hydrolysis rate was lower than glucose decomposition rate. Kamio et al.15 reported that cellulose hydrolysis drastically increased above 240 °C and that the hydrolysis reaction started by an initial formation of oligosaccharides, which were subsequently converted into monosaccharides and secondary products. Mok and Antal16 reported complete hemicellulose solubilisation when different biomasses were treated at 230 °C and 34.5 MPa for 2 minutes. Sasaki et al.14 analysed the decomposition of xylose at temperatures between 360–420 °C and pressures comprising 25 and 40 bar using very short reaction times (0.02–1 s). They found that the retro-aldol condensation reaction was one of the most important reactions. Jeon et al.11 analysed the hydrothermal treatment of alginic acid, examining the effect of the reaction time (0–40 min) and temperature (160–220 °C) using various metal cations. Furfural was the major reaction product, its yield initially increased with time and then decreased due to the conversion of furfural to humins and/or organic acids. The same authors12 reported a maximum furfural yield occurring when 0.5 wt% alginic acid was hydrothermally treated with 600 mg of a Amberlyst-15 catalyst at 180 °C for 30 minutes.
The use of microwave heating has recently appeared as a promising alternative for the selective and controllable pre-treatment of biomass. Several processes have been addressed for biomass pyrolysis,9,17–24 hydrothermal depolymerisation,17,18,22,25–30 fractionation and solvolysis.18,31–38 Microwave heating is based on the high frequency rotation of polar molecules, which produces a quicker and higher heating of the species with high polarity.17–19,25–27,33,39 As lignin has a lower polarity than cellulose and hemicellulose, it is less active during microwave heating. This allows the solubilisation of the carbohydrate content of the biomass without significantly solubilising the lignin fraction.35,40 This minimises the presence of lignin-derived inhibitors in the hydrolysate. However, it must also be borne in mind that the intensive hydrogen bonds within the cellulose structure together with its high crystallinity hinder the depolymerisation of cellulose41,42 during microwave heating, which also decreases the reactivity of this carbohydrate in comparison to hemicellulose or alginic acid. However, a possible solution when working with lignocellulosic biomass might be the addition of a catalyst, such as acetic acid, which allows the solubilisation of the cellulose and hemicellulose without dissolving its lignin content.40 Then, acetic acid could be separated from the hydrolysate prior to fermentation and used again in further reactions.40 In addition, as water is highly effective in microwave energy absorption, the combination of hydrothermal conditions together with microwave assisted heating might result in a very promising technology to achieve a selective and controllable production of saccharide-rich aqueous solutions from biomass.
However, publications addressing the use of microwaves to achieve hydrothermal conditions for the depolymerisation of biomass carbohydrates are rare. Fan et al.26 addressed the microwave-assisted depolymerisation of cellulose between 150 and 270 °C, making comparison between microwave and conventional heating. Higher process efficiency, controllability and sugar selectivity were achieved with the microwave-assisted method than with the conventional hydrothermal treatment. In addition, the liquids produced with microwave heating had a higher sugar/inhibitors ratio along with a particularly high glucose selectivity. Jiang et al.43 analysed the microwave-assisted, NaCl catalysed, hydrothermal depolymerisation of xylo-oligomers, examining the effect of the temperature (140–200 °C) and reaction time (0–60 min). An increase in the temperature and/or reaction time increased the depolymerisation rate and the concentration of sugars in the hydrolysate. Wang et al.44 analysed the production of value-added products from alginic acid using different copper salts in various aqueous biphasic systems at 200 °C for a reaction time as short as 1 min. They reported furfural yields ranging from 28 to 31% at optimum operating conditions.
These publications provide valuable insights into the behaviour of different biomass structural components alone. Nonetheless, since different reactors, heating mechanisms and operating conditions were used, the comparison between the reactivity of the different carbohydrates when subjected to hydrothermal conditions is unreliable. Furthermore, the feasibility of using these liquids in subsequent fermentation processes was not addressed. Herein, this work addresses the microwave-assisted hydrothermal treatment of cellulose, hemicellulose and alginic acid for the production of saccharide-rich solutions. The reactivity of the biomass carbohydrates and the fermentability of the liquids produced were thoroughly analysed. In the reactivity analyses, the influence of the temperature, reaction time and solid to water ratio was investigated on the yield and chemical composition of the liquids produced. In addition, the effects of the carbohydrates nature and hydrolysates chemical composition on the fermentation results was investigated using the robust yeast Metschnikowia pulcherrima (MP). This yeast was selected due to its ability to metabolise a wide range of mono-/di-saccharides and water-soluble oligosaccharides. In addition, it also possesses a high tolerance to inhibitors, such as furfural, 5-HMF and organic acids. One of the products that MP is capable of producing is 2-phenylethanol, a high value fragrance molecule.45
The fact that the reactivity of the most representative biomass carbohydrates when subjected to the same hydrothermal treatment (conventional or microwave-assisted) has never been compared under the same conditions before demonstrates, along with the fermentability analyses conducted examining the effects of the chemical composition of the liquids on the fermentation results, that this work represents a novel and challenging investigation in this field supporting the development of new thermochemical and biological integrated routes.
2. Experimental
2.1 Materials
Cellulose and alginic acid were purchased from Sigma Aldrich. Xylan extracted from beech wood (cell wall polysaccharide, >90% xylose residues), was purchased from Serva Electrophoresis GmbH. All the carbohydrates were used as received.2.2 Microwave experiments
The effects of the temperature (160–210 °C), reaction time (0 and 5 min) and solid/water (S/W) ratio (1/20 and 1/10) were analysed and compared for cellulose, hemicellulose and alginic acid. A temperature lower than 220 °C and a time shorter than 5 min were used in order to study the reactivity of these carbohydrates under conditions at which the depolymerisation of the carbohydrate fraction is preferential over the lignin fraction to gain useful insights for future investigations with real biomass. In addition a holding time of 0 min (i.e. only the 15 min of ramping time needed to reach the reaction temperature) was also analysed to study the effect of the heating-up step. The experiments were carried out in a CEM-Mars microwave system using a 70 mL scale PrepPlus® reactor copped with a fibre optic temperature sensor. For the microwave experiments, different amounts of solid (cellulose, hemicellulose or alginic acid) together with water (40 mL) were loaded into the microwave reactor depending on the solid/water ratio used in each experiment. Then the reactor was closed and placed inside the microwave unit. A ramping time (time to reach the reaction temperature) of 15 min was used for all the experiments. After reaction, the reaction mixture was cooled to room temperature by CEM-Mars default programme, centrifuged, and the solid residue (pellet) was isolated and dried (105 °C). The resultant solid (pellet) and the supernatant (hydrolysate) were stored for further analyses.2.3 Fermentation
Inoculum cultures for M. pulcherrima were prepared by culturing in 10 mL SPME media (soy peptone: 30 g L−1; malt extract: 25 g L−1) and 10 mL YPD media (yeast extract: 10 g L−1; peptone: 20 g L−1; glucose: 20 g L−1) and incubated at 20 °C using an agitation speed of 180 rpm for 24 h. The hydrolysates from the microwave processing stage were filtered, adjusted to pH 4 (be means of a 2 mol L−1 NaOH solution) and diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with a salt solution. This salt solution was made up of KH2PO4 2 g L−1; MgSO4·7H2O 0.376 g L−1; MgCl2·6H2O 2.16 g L−1; ZnSO4·7H2O 0.04 g L−1, (NH4)2SO4 0.126 g L−1; NH4Cl 0.708 g L−1; yeast extract 2.00 g L−1; CaCl2·2H2O 0.300 g L−1, tartaric acid 30.0 g L−1. Cultures of 1 mL were inoculated with 25 μL of inoculum in 24-well plates and incubated at 180 rpm at 20 °C for 168 h. Throughout the fermentation the growth of the cultures was estimated for absorbance at 600 nm and the biomass was recovered by centrifugation in 1.5 Eppendorf tubes (10
2 with a salt solution. This salt solution was made up of KH2PO4 2 g L−1; MgSO4·7H2O 0.376 g L−1; MgCl2·6H2O 2.16 g L−1; ZnSO4·7H2O 0.04 g L−1, (NH4)2SO4 0.126 g L−1; NH4Cl 0.708 g L−1; yeast extract 2.00 g L−1; CaCl2·2H2O 0.300 g L−1, tartaric acid 30.0 g L−1. Cultures of 1 mL were inoculated with 25 μL of inoculum in 24-well plates and incubated at 180 rpm at 20 °C for 168 h. Throughout the fermentation the growth of the cultures was estimated for absorbance at 600 nm and the biomass was recovered by centrifugation in 1.5 Eppendorf tubes (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min). The supernatant and the pellets were stored separately at −20 °C for further analyses.
000 rpm, 5 min). The supernatant and the pellets were stored separately at −20 °C for further analyses.
2.4 Response variables, analytical methods and statistical analyses
Several response variables were used to analyse the effect of the microwave operating conditions on the process (Table 1). These include the overall carbohydrate conversion, the gas and liquid yields, the liquid carbon efficiency (C-wt%) and the chemical composition (in carbon basis, C-wt%) of the hydrolysates. The gas phase composition could not be measured, as the reactor system is not designed for gas analysis. In addition, the biomass growth and 2-PE yield produced during fermentation were used to study the suitability of the liquid samples for fermentation. The liquid phase (hydrolysate) was analysed by High-Performance Liquid Chromatography (HPLC) and Total Organic Carbon (TOC). The HPLC analyses were conducted using an Agilent 1260 apparatus fitted with an Infinity II RI Detector using two different columns. Sugars and carboxylic acids were determined with an Agilent Hi-Plex H (7.7 × 300 mm, 8 μm (p/n PL1170-6830) column, using a 0.005 M H2SO4 solution as the mobile phase. Mono-/di-saccharides include cellobiose, xylose, glucose, fructose, mannose, arabinose, rhamnose and levoglucosan. Oligosaccharides comprise DP 2–6 cellu- and xylo-saccharides: cellobiose and xylobiose (DP2), cellotriose and xylotriose (DP3), cellotetraose and xylotetraose (DP4), cellopentaose and xylopentaose (DP5) and cellohexaose and xylohexaose (DP6). The proportion of DP > 6 oligomers was estimated as the difference between the total amount of carbon in the solution determined by TOC and the total amount of carbon quantified by HPLC. It is believed that the vast amount of this unidentified carbon is accounted for by high molecular weight oligomers produced during the depolymerisation, although other species such as humins could slightly contribute to this fraction. Carboxylic acids comprise lactic, formic, levulinic, acetic, guluronic, and mannuronic acids. Besides, an ACE C18 (250 × 4.6 mm, 5 μm particle size) column was used to determine the amounts of ketones and furans (levoglucosenone, 5-HMF and furfural, respectively), employing an acetonitrile/water (25/75, vol/vol) solution as the mobile phase. A liquid flow rate of 0.8 mL min−1 was used for both columns. TOC analyses were conducted in a Vario TOC Cube Analyser. FTIR data was produced using a PerkinElmer FTIR/FTNIR Spectrum 400 analyser. The spectra were acquired between 700 and 4000 cm−1 with steps of 2cm−1. Optical Density (absorbance at 600 nm using a corrector internal factor) was used to estimate the biomass yeast growth. GC-FID was used for the quantification of the 2-PE.One-way analysis of variance (one-way ANOVA) with the multiple range Fisher's least significant difference (LSD) test, both with a significance level of 0.05, were used to evaluate the influence of the carbohydrate type, temperature, solid/water ratio and reaction time on the process. In the figures, these effects have been graphically represented for different temperatures (160, 190 and 210 °C) using two different reaction times (0 and 5 min) for a S/C ratio of 1/20. In addition, the effect of the S/C ratio has been plotted at different temperatures (160, 190 and 210 °C) for a reaction time of 0 min. The results of the ANOVA analyses are provided as p-values. P-Values lower than 0.05 indicate that at least two values are significantly different. Furthermore, the LSD test was used to compare pairs of data. The results of the LSD tests are presented graphically in the form of LSD bars. To ensure significant differences between any pair of data, their LSD bars must not overlap. A multivariate analysis by means of Spearman's test was conducted to find evidence for the relationships between the chemical composition of the hydrolysates and the fermentation results. In addition, the fermentation results were correlated to the chemical compositions of the hydrolysates using statistical linear additive models with 2 level linear interactions. The Akaike Information Criterion (AIC) was used to choose the best model. The AIC is intended to help select the best model from among several competing models. This model should strike a balance between fitting the data well and using only a few parameters, allowing the identification of the chemical compounds and interactions with the highest probability of being responsible for the different fermentability of the hydrolysates.46–48 Then, the relative influence (positive and negative) of these species was calculated using the Cause-Effect Pareto Principle. More information about these statistical tools can be found in the ESI.†
3. Results and discussion
3.1 Global conversion and yields to gas and liquid products
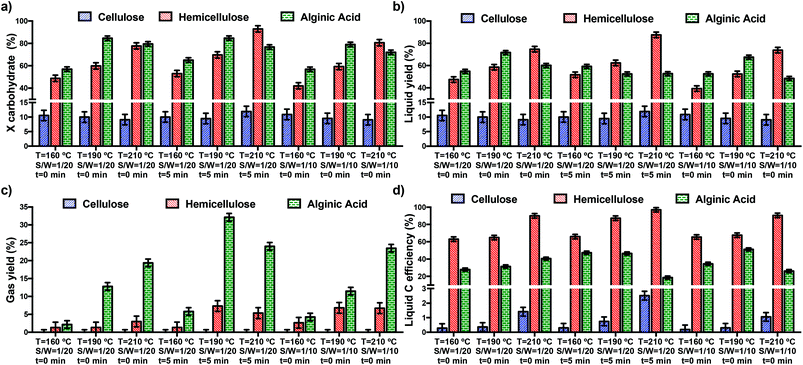
Fig. 1 shows the overall carbohydrate conversion, the liquid and gas yields and the liquid C efficiency obtained during the microwave-assisted experiments. The statistical analysis reveals significant differences between the results obtained in the experiments (p-values < 0.001). In addition, the Fisher's LSD test shows that both the type of carbohydrate and the operating conditions (temperature, time and S/W ratio) exert a significant influence on the process with 95% confidence. This indicates that the carbohydrates tested in this work have different reactivities when subjected to microwave heating.Cellulose displays a very low reactivity. In particular, gas formation is negligible and the overall carbohydrate conversion and liquid yield and C efficiency are lower than 10% in all the cases. This is the consequence of the relatively low temperature and short reaction times used in the experiments. Under these conditions, only the amorphous part of cellulose (around 10 wt%) is solubilised in the liquid when subjected to microwave-assisted hydrothermal treatment.27 Conversely, hemicellulose and alginic acid show a much higher reactivity under the same experimental conditions and the operating conditions exert a statistically significant influence on the hydrothermal treatment of hemicellulose and alginic acid and different outcomes are observed. For hemicellulose, an increase in the temperature between 160 and 210 °C leads to an increase in the overall carbohydrate conversion and liquid yield and C efficiency regardless of the S/W ratio or reaction time. An increase in the temperature positively influences the kinetics of the process.4 Under conventional hydrothermal treatment, hemicellulose depolymerisation starts at around 170 °C;4 however, conversions higher than 50 wt% take place using microwave-assisted hydrothermal depolymerisation, thus highlighting the efficiency of microwave heating for the hydrothermal conversion of biomass.19,33,35,49 The effect of the reaction time depends on the temperature. In particular, an increase in the reaction time from 0 to 5 min increases the overall carbohydrate conversion and the liquid yield when temperatures between 190 and 210 °C are used due to the longer exposure of the material to microwaves. In these cases, the higher the temperature, the greater is the positive effect of the reaction time on the process. An increase in the temperature exerts a positive kinetic effect on the process and therefore, lower reaction times are needed to achieve the same level of conversion. This relationship between the temperature and the reaction time was defined as severity (temperature × time) in the work of Prado et al.4 They also found that an increase in temperature shifted the maximum liquid yield towards a lower residence time due to the decrease in the severity of the process. It is also important to note that gas formation is very low (<5%) in all the cases; thus maximising the selectivity towards liquid production. The highest gas production takes place when elevated temperatures (>190 °C) along with either long (5 min) reaction times or low S/W ratio are used. These conditions can promote decarboxylation reactions leading to gas formation.50,51 In general the S/W ratio does not significantly influence the overall carbohydrate conversion. An exception to this occurs when high temperature and low S/W ratios are used. These conditions produce a small decrease in the liquid yield due to the increase occurring in the gas yield.
As regards alginic acid depolymerisation, an initial increase in the temperature from 160 to 190 °C increases the carbohydrate conversion; however, a further increase up to 210 °C leads to decrease in the conversion regardless of the S/W ratio or reaction time. While the initial increase with the temperature is the consequence of the positive kinetic effect of this variable on the process as described earlier,4 the subsequent decrease is accounted for by the formation of solid species, such as char and humins, at high temperature.52–54 The formation of these macromolecules can occur from the furans produced during the decomposition of alginic acid via aldol addition followed by condensation or polymerisation.55–61 Therefore, as the overall solid conversion was calculated by mass difference, an increase in the formation of these insoluble solid compounds produces an “artificial” decrease in this value. The effect of the S/W ratio and reaction time on the overall conversion depends on the temperature. While an increase in either the time or S/W ratio does not influence the overall conversion at 160 °C, a decrease is observed between 190 and 210 °C due to the formation of humins when high temperature or long reaction times are used.52–54
The operating conditions also have a significant influence on the liquid yield and liquid C efficiency. In particular, regardless of the S/W ratio, an increase in the temperature when a very short (0 min) reaction time is used has the same consequences for the liquid yield and C efficiency as those described above for the overall conversion; i.e. an initial increase between 160 and 190 °C followed by a subsequent decrease between 190 and 210 °C. However, an increase in the reaction time from 0 to 5 min statistically decreases the liquid yield and C efficiency and modifies the effect of the temperature on this variable. For a 5 min reaction time, an increase in the temperature from 160 to 210 °C leads to a progressive depletion in the liquid yield and C efficiency. This decrease is the consequence of the increase in gas production and solid formation when high temperatures and long reaction times are used.4 Therefore, gas and solid formation results in lower liquid yields. In particular, at medium temperature (190 °C), the decreases in the liquid yield and C efficiency are accounted for by gas formation,4 while at high temperature (210 °C) solid formation is responsible for the decrease observed in the liquid yield.52–54 The effect of the S/W ratio on the depolymerisation of alginic acid depends on the temperature. At 160 °C the S/W ratio does not have a significant influence, while an increase in the S/W ratio from 1/20 to 1/10 decreases the overall alginic acid conversion between 190 and 210 °C. This lower overall conversion is the consequence of the greater gas production and solid formation occurring when long reaction times and high temperatures are used. An increase in the S/W ratio increases the solid/microwave ratio; thus increasing the severity of the process. This shifts alginic acid decomposition towards the formation of final products; i.e. humins and gases.
Gas formation is substantial during the hydrothermal treatment of this carbohydrate and gas yields as high as 30% are obtained in some cases. This is in good agreement with the reaction pathway shown in Fig. S1.† For this carbohydrate, sugars production implies gas formation via decarboxylation. This is in good agreement with the lower C efficiency of alginic acid than that of hemicellulose due to the much lower C content in the liquids produced from the former than from the latter due to the formation of CO2via decarboxylation. For a 0 min reaction (i.e. only the 15 min ramping without holding), regardless of the S/W ratio, an increase in the temperature from 160 to 210 °C lead to a substantial increase in the gas yield. In these cases, the effect of the S/W ratio is not very important. Though, the reaction time has a more important influence on gas formation; an increase from 0 to 5 min substantially increases gas formation, especially at medium temperatures. In these cases, an increase in the residence time shifts the reaction towards the formation of low oxygen content liquid products, which are easily converted into gases under the conditions used in this work. This increase occurring when the reaction time increases from 0 to 5 min is less pronounced at 210 °C than at 190 °C. For a 5 min reaction an increase in the temperature from 190 °C to 210 °C leads to a substantial decrease in gas formation due to the formation of solid products; i.e. humins and char, when high temperatures and long reaction times are used.52–54
With respect to carbohydrates reactivity, it increases as follows: cellulose < hemicellulose < alginic acid. However, and very importantly, the higher reactivity of alginic acid than that of hemicellulose does not produce higher liquid (hydrolysate) yield under some conditions due the substantial formation of gas and solid products during the hydrothermal treatment of alginic acid. This different reactivity is the consequence of the different structure of these carbohydrates, which results in different behaviours when subjected to microwave heating. The greater proportion of side-groups, the less uniform structure, and lower polymerisation degree of hemicellulose and alginic acid than cellulose10 are the major factors responsible for the different reactivity of these carbohydrates. In addition, the acidic functionalities of alginic acid, and the high formation of acids, can promote the autocatalytic depolymerisation of this carbohydrate.
3.2 Chemical composition of the liquids
The liquid product (hydrolysate) consists of a mixture of oligo- (DP 3–6 and DP > 6) and mono-/di-saccharides, carboxylic acids, ketones and furans. Mono-/di-saccharides include cellobiose, xylose, glucose, fructose, mannose, arabinose, rhamnose and levoglucosan. Carboxylic acids comprise lactic, formic, levulinic, acetic, guluronic, and mannuronic acids. Ketones and furans are made of levoglucosenone, 5-HMF and furfural, respectively. The presence of these compounds in the liquids is in good agreement with the reaction pathways shown in Fig. S1.† The chemical composition in carbon basis (C-wt%) of the hydrolysates produced during the hydrothermal treatment of cellulose (a and b), hemicellulose (c and d) and alginic acid (e and f) is shown in Fig. 3. The statistical analysis of the results by means of the ANOVA and Fisher's LSD tests reveals significant differences between the results obtained in the experiments (p-values < 0.001). The type of carbohydrate and the operating conditions exert a significant influence on the chemical composition of the liquids.As regards the chemical composition of the hydrolysates produced from cellulose, DP > 6 oligosaccharides are the most abundant species in the liquid phase, followed by monosaccharides (largely glucose, fructose and mannose) and in much lower proportions furans, carboxylic acids and ketones. While the operating conditions exert a statistically significant influence on the proportions of oligo- and monosaccharides, the concentrations of furans, carboxylic acids and ketones are not significantly affected. In addition, the proportions of these latter compounds are lower than 8 C-wt% in all cases. This suggests that the secondary reactions leading to the formation of these compounds did not occur to a great extent, thus highlighting the good selectivity and controllability of microwave-assisted heating.35 Regardless of the other conditions, an increase in the reaction time decreases DP > 6 oligosaccharides and increased mono- and disaccharides, as a proportion in the liquid, respectively. This supports the evidence that the vast amount of the unidentified C accounts for the formation of DP > 6 Oligossacharides. An increase in the temperature promotes a further development of the hydrolysis reactions; thus leading to the progressive depolymerisation of the DP > 6 oligosaccharides fraction into DP 3–6 oligosaccharides, disaccharides and monosaccharaides.4 In addition, these reactions are intensified by the reaction time and a greater decrease in the proportions of DP > 6 oligosaccharides occurs when the temperature is increased from 160 to 190 °C for a 5 min reaction than for a 0 min reaction. This is a consequence of the positive effect that the reaction time and the temperature exerts on the kinetics of the process.4 Furthermore, the increases occurring for the proportions of DP 2–6 oligosaccharides and saccharides are also intensified. As a result of this, the liquid has a relatively high proportion of saccharides when high temperatures and long reaction times are used.
The S/W ratio has a much lower, although significant, influence on the proportions of oligosaccharides, disaccharides and monosaccharides. Specifically, an increase in the S/W ratio from 1/20 to 1/10 decreases the concentration of DP > 6 oligosaccharides and increases the concentration of DP 2–6 and mono-/di-saccharides. An increase in the S/W ratio increases the solid/microwave energy ratio; thus augmenting the amount of microwave energy which is effectively absorbed by the species solubilised in the liquid fraction. This development promotes a further extension of the hydrolysis and depolymerisation reactions, thus increasing the amount of monosaccharides in the solution.
With respect to hemicellulose, the liquid phase contains a great proportion of DP > 6 oligosaccharides (>90 C-wt%). In addition, the operating conditions do not exert a significant influence on the composition of the liquid phase in most of the cases. One exception occurs when a long reaction time (5 min) or high temperatures are used. In this case, an increase in the temperature from 190 to 210 °C slightly decreases the proportion of DP > 6 oligosaccharides and increases the proportion of saccharides. Therefore, at 210 °C, an increase in the reaction time from 0 to 5 min leads to a decrease in the proportions of DP > 6 oligosaccharides and an increase the proportion of saccharides. These variations are accounted for by the positive kinetic effect of the reaction time on the depolymerisation and hydrolysis reactions at high temperature.4 The proportions carboxylic acids, furans and ketones are very low (<5 C-wt%). For these latter compounds, neither the temperature nor the reaction time or the S/W ratio exerts a significant influence on their concentrations. These developments suggest that under the operating conditions tested in this work, the initial depolymerisation of hemicellulose into soluble oligosaccharides might be faster than their subsequent decomposition of into monosaccharides and organic compounds, such as furans and carboxylic acids. This allows a good balance between monosaccharide production and degradation to be achieved; which is of paramount importance for the production of a fermentable broth.
The liquid phase produced from alginic acid is made up of a complex mixture of several organic compounds. Not only saccharides, but also products from secondary reactions, such as carboxylic acids and furans which are present in high quantities in the hydrolysates. In addition, and unlike what was described for cellulose and hemicellulose, the operating conditions play a very important role in the chemical composition of the hydrolysates. Regardless of the S/W ratio (1/20 or 1/10), at low temperature and short reaction time (160 °C and 0 min), the liquid phase contains a high proportion of DP > 6 oligomers/oligosaccharides, DP 3–6 oligosaccharides, disaccharides and monosaccharides; the total concentration of carboxylic acids, furans and ketones being quite low (<15 C-wt%). At low temperatures, the process is not shifted towards the production of low oxygen content liquid products due to the very short reaction time used in this work (<5 min) and therefore, the production of secondary products does not occur to a great extent.
Though, an increase in the temperature promotes the hydrolysis and depolymerisation reactions, which results in different evolutions for the chemical composition of the liquids depending on the S/W ratio and the reaction time. For a low S/W and short time (1/20 and 0 min), the composition of the liquids depends on the temperature with different trends being observed. On the one hand, the relative amounts of DP 2–6 saccharides and ketones decreases and increases, respectively, when the temperature increases from 160 to 210 °C. However, the concentration of ketones is very small and this variation is not very important from a practical point of view. On the other hand, maxima and minima occur for the proportions of DP > 6 oligomers/oligosaccharides, di-/monosaccharides, carboxylic acids and furans. Between 160 and 190 °C, the proportion of DP > 6 oligomers/oligosaccharides decreases, while concentrations of monosaccharides, carboxylic acids and furans increase. Conversely, between 190 and 210 °C, the concentration of DP > 6 oligomers/oligosaccharides increases and the proportions of monosaccharides, carboxylic acids and furans decrease. The positive kinetic effect of the temperature promoting a further development of the hydrolysis reactions accounts for the progressive transformation of DP > 6 oligomers/oligosaccharides into guluronic/mannuronic acids and DP 2–6 saccharides into monosaccharides between 160 and 190 °C. A further increase in the temperature up to 210 °C shifts the process towards the formation of liquid end products such as furans and short chain carboxylic acids.11,12,44 This is in good agreement with the increase observed in gas production and the decrease in the relative amount of carboxylic acids in the liquid. This decrease for carboxylic acids is accounted for by the lower proportion of guluronic and mannuronic acids in the hydrolysates as well as for the transformation of some of the organic species into gases. This also explains the small increase occurring for the proportion of DP > 6 oligomers/oligosaccharides in the liquid (Fig. 2).
 | ||
| Fig. 3 FTIR spectra of the original carbohydrates and spent solids produced from cellulose (a–c), hemicellulose (d–f) and alginic acid (g–i) under different conditions. | ||
The S/W ratio also exerts a significant influence. Precisely, an increase from 1/20 to 1/10 modifies the effect of the temperature on the process. For a S/W ratio of 1/10 the proportion of DP > 6 oligomers/oligosaccharides is not substantially affected by the temperature and the liquid has a high concentration of these compounds (around 50 C-wt%) in all the cases. Increasing the temperature from 160 to 210 °C decreases the concentrations of DP 2–6 saccharides and carboxylic acids and slightly increases the relative amounts of furans and ketones. These variations are caused by the higher solid/microwave energy ratio occurring when the S/W ratio increases, which promotes the advancement of the reactions occurring in the liquid phase towards the formation of carboxylic acids and furans.11,12,44 The decrease observed for the amounts of guluronic/mannuronic acids is higher than the increase occurring in the proportion of formic acid, and therefore, the total amount of carboxylic acids in the liquid decreases. In addition, the positive effect of the S/W ratio, leading to a greater advancement of the chemical reactions taking place in the liquid phase, correlates well with the increase observed in the proportion of furans.
The effect of the reaction time strongly depends on the temperature. At 160 °C, an increase in the reaction time from 0 to 5 min, substantially decreases the concentration of DP 3–6 oligosaccharides and increases the proportions of DP > 6 oligomers/saccharides and carboxylic acids. The reaction time promotes the transformation of DP 2–6 oligosaccharides into smaller saccharides. In addition, the concentration of guluronic and manuronic acids in the liquid also increases due to the transformation of the water-soluble oligomers into these carboxylic acids. However, the increase observed in the relative amount of carboxylic acids is not as pronounced as it might have been expected, probably due to the subsequent transformation of some carboxylic acids obtained in the final steps into gases when long reaction times used. This gas formation also accounts for the small increase observed in the proportion of DP > 6 oligomers/oligosaccharides. The formation of gas occurring when increasing the reaction time increases the relative amount of these species in the liquid phase.
At 190 °C gas formation is more pronounced, which indicates that the transformation of some of the liquid species into gases occurs to a greater extent. In particular, at 190 °C, an increase in the reaction time promotes the transformation of guluronic/mannuronic acids into saccharides and these could subsequently be transformed into furans, formic acid and levulinic acid. However, these smaller acids can also decompose to produce gas. This development results in an overall decrease in the concentration of carboxylic acids together with an increase in the proportion of DP > 6 oligomers/oligosaccharides due to the removal of some species from the liquid phase. These reactions occur to a greater extent when the temperature increases. As a result, at 210 °C, an increase in the reaction time significantly increases the concentration of furans due to the advancement of the reactions in the liquid phase leading to the formation of less oxygenated liquid products. This effect is in good agreement with the increase in solid production observed at higher temperatures and longer reaction times as humins and char can be produced from furfural and 5-HMF.52–54
The results described above indicate that the nature of the carbohydrate exerts a very important influence on the chemical composition of the liquid hydrolysates. The liquid phase obtained from alginic acid contains a high amount of products from secondary reactions, such as carboxylic acids, furans and ketones due to the higher reactivity of this carbohydrate under the operating conditions. This is in good agreement with the high overall conversion and liquid and gas yields obtained during the treatment of this carbohydrate. Conversely, cellulose and hemicellulose display a much lower reactivity and the formation of these organic compounds does not take place to a significant extent. This resulted in a liquid phase containing a greater proportion of DP > 6 oligosaccharides, DP 3–6 oligosaccharides, disaccharides and monosaccharides. Despite the lower reactivity of cellulose than hemicellulose, the relative amounts of DP 3–6 oligosaccharides and smaller saccharides are higher for the former than for the latter. This implies that under the operating conditions tested in this work, the amorphous part of cellulose is more reactive than hemicellulose. In addition, the ratio between the microwave active species solubilised in the liquid and the total microwave power ratio is higher for cellulose than for hemicellulose; thus increasing the reaction rate of the hydrolysis reactions occurring in the liquid phase, this explains the differences observed in the liquid composition for both carbohydrates.
3.3 Characterisation of spent solids produced in the experiments
To gain a deeper insight into the reactivity of the different carbohydrates, the original and spent solids from the microwave experiments were characterised by means of FT-IR. The spectra obtained from the original and spent solids produced from cellulose (a–c), hemicellulose (d–f) and alginic acid (g–i) are given in Fig. 4. The FT-IR spectra of the original carbohydrates are in good agreement with those previously reported in the literature.62,63 For cellulose and hemicellulose, the strongest bands can be seen in the range between 1034 and 1112 cm−1, which correspond to –C–O vibrations assigned to sugars.62 In addition, for hemicellulose the vibrations for the β-glycosidic bonds are also important.62 In the case of alginic acid, the strongest vibrations occur at 1219 and 1230 cm−1, due to the presence of –C–O bonds, and at 1738 cm−1, which accounts for the carboxylic acid functionalities (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of this carbohydrate.63
O) of this carbohydrate.63
The comparison between the spectra of the original carbohydrates and the spent solids can help to gain more insight into the different reactivity of these materials. In particular, while there are not substantial differences between the spectra of the original and the spent solids for cellulose, some differences occur for hemicellulose and alginic acid. The lack of differences occurring in the case of cellulose might indicate that the core structure of the solid was unaffected during the microwave treatment. This supports the hypothesis that only the amorphous part of the cellulose interacted with microwaves and explains the low reactivity of this material at the operating conditions used in this work. The significant variations occurring for hemicellulose and alginic acid are the consequence of the higher reactivity of both solids. When they are subjected to microwave-assisted treatment, their structures are modified due to their progressive solubilisation into the liquid phase.
In particular, for hemicellulose the major change occurs for the band at 1042 cm−1. The bands between 1152 and 995 cm−1 are typical of arabinoxylan, attributing to the stretching and bending vibrations of C–O, C–H and C–OH.64 An increase in either the temperature or the reaction time during the microwave treatment increases the intensity of this band in the spent solids. However, these variations are probably not structurally important since the spectra are not modified substantially in comparison with the original spectrum for hemicellulose. This suggests that the progressive depolymerisation of hemicellulose occurs without a significant alteration of the core structure of the solid; i.e. a sequential depolymerisation to oligomers, followed by a further depolymerisation to water-soluble oligomers without substantially modifying the monomeric units in the macromolecular structure of the solid. Conversely, greater variations are observed between the original alginic acid and the solids produced from this carbohydrate. The major difference occurs at 210 °C, the temperature at which the solid displays a substantial change in its structure. In addition, the most characteristic band of this material (1030 cm−1) disappears, probably due to the high conversions achieved at high temperature, and a new band appears at a 1705 cm−1. Interestingly, this new band is characteristic of the humins produced from sugars;65 thus providing good evidence of the formation of these macromolecules when alginic acid is treated at high temperatures and/or long reaction times.
3.4 Suitability for fermentation
The hydrolysates produced by the microwave reaction contained mainly oligosaccharides, a range of C5 and C6 sugars and inhibitors. We recently reported that the yeast M. pulcherrima has excellent inhibitor tolerance and can metabolise a wide range of saccharide materials.36 To assess the effect of the MW processing conditions on the fermentability of the hydrolysate from the three carbohydrates, M. pulcherrima was cultured in 24 well plates on the hydrolysate with additional N, P and microelements added. The yeast biomass growth is shown in Fig. 4. These results were also standardised by the amount of hydrolysate present in order to establish a reliable comparison of the fermentability of the solubilised fraction. The statistical analysis of the results reveals that the type of carbohydrate and the microwave operating conditions have a significant influence (p-value < 0.001) on the fermentation results. In addition, a multivariate analysis by means of Spearman's test was conducted to find evidence for the relationships between the chemical composition of the hydrolysates and the fermentation results. This analysis reveals significant relationships for the standardised biomass production (g/g) with the proportions of DP > 6 oligomers (p-value = 0.13; R2 = 0.35), saccharides (p-value = 0.05; R2 = 0.17), carboxylic acids (p-value = 0.001; R2 = 0.72), ketones (p-value = 0.001; R2 = 0.46) and furans (p-value = 0.028; R2 = 0.19). In addition, the standardised 2PE production (g/g) statistically depends on the relative amounts of DP 3–6 Oligomers (p-value = 0.14; R2 = 0.16), saccharides (p-value = 0.14; R2 = 0.16) and furans (p-value = 0.001; R2 = 0.45). The low Sperman's regression coefficient (R) occurring for some species indicates that the relationship is not linear, even thought is statistically significant.Overall, the hydrolysates produced from cellulose produce the highest concentration of yeast biomass growth at high temperatures and loadings, and give the most fermentable material over all conditions tested. While at S/W loadings of 1/20, more yeast biomass in total is produced from the hemicellulose fractions there was far higher solubilised material available for the yeast, and therefore the conversion is relatively poor. Oligosaccharides from alginic acid were the least fermentable. These differences potentially are the result of the different chemical composition of the liquids obtained from each carbohydrate, with the cellulose producing simple straight chain units as opposed to highly branched oligosaccharides from hemicellulose;66–68 and alginic acid hydrolysates containing high proportions of carboxylic acids and furans. Previous studies with M. pulcherrima reported that this yeast is capable of depolymerising long chain water-soluble oligosaccharides into short chain oligosaccharides; these latter being subsequently assimilated.36 As described earlier, an increase in the S/W ratio decreases the amount of DP > 6 oligosaccharides in the liquids, producing a more accessible hydrolysate. Alternatively, alginic acid hydrolysates contained a particularly high level of inhibitors whereas there was negligible concentrations of inhibitors such as carboxylic acids and furans in the cellulose hydrolysates. A similar concentration of yeast biomass was produced for every hemicellulose depolymerised material. Though the chemical composition of the liquid produced from hemicellulose is not significantly affected by the operating conditions.
M. pulcherrima is known to produce high levels of 2PE, with up to 1.5 g L−1 reported from high concentrations of glucose (100 g L−1 sugar loading).36,45,69 For all the biomass carbohydrates, the production of 2PE is significantly affected by the hydrothermal conditions used in the microwave experiments (Fig. 4). For cellulose, little 2PE is produced from the oligosaccharides, with the highest productivity being observed with the highest yeast biomass and solubilised material present. For hemicellulose, the highest 2PE production occurs during the fermentation of the liquids produced at low temperature (160 °C). Regardless of the reaction time or S/W ratio, an increase in the temperature of the microwave treatment from 160 to 210 °C leads to a substantial decrease in the production of 2PE. In addition, this decrease is more marked when either long reaction times or high S/W ratios are used. Similarly, the highest production of 2PE is observed for alginic acid. Regardless of the S/W ratio or reaction time, an increase in the temperature from 160 °C to 190 °C significantly increases the production of 2PE, where a maximum is achieved. A further increase up to 210 °C however leads to a decrease in the production of 2PE. This suggests that similarly to previous studies, a higher concentration of material is necessary to produce high yields of 2PE. Therefore, the higher standardised fermentability of cellulose did not translate into a higher 2PE production; hemicellulose and alginic acid being more suitable materials for the production of this high value compound due to the higher liquid C efficiency achieved with these two carbohydrataes.
Fig. 5 shows the chemical compounds and interactions between compounds with the highest probability of being responsible for the different fermentation results of the hydrolysates according to the AIC and cause-effect Pareto principles. Fig. 5a reveals that carboxylic acids followed by furans exert the highest negative impact on the standardised biomass production, while saccharides and Oligomers DP > 6 have a positive influence. HMF and furfural are reported to be some of the most toxic inhibitors to most yeasts, especially in combination. These inhibitors disrupt cell growth by reducing the activity of various enzymes involved in respiration, damaging DNA and in so doing disrupt DNA and RNA synthesis.66–68Fig. 5b reveals that the standardised production of 2PE is positively influenced by the presence of carboxylic acids, furans and ketones in the hydrolysates. Conversely, the interactions of ketones with furans and Oligomers DP 3–6 as well as the presence of Oligomers DP 3–6 in the solution negatively influence the standardised production of 2PE. Like a number of oleaginous yeast, Metschnikowia pulcherrima is capable of converting furan compounds producing a less toxic substrate; thus allowing the potential assimilation of furan compounds and sugars to produce 2PE.36 This can increase the selective production of 2PE for hydrolysates containing a high proportion of dissolved C. In this regard, as described above, the production of 2PE increases with increasing the amount of C in the solution,45 which explains the higher production of 2PE achieved with hemicellulose and alginic acid hydrolysates than that obtained with the liquids produced from cellulose. The results of this statistical analysis help to explain the higher fermentability of cellulose and hemicellulose hydrolysates in comparison with the liquids produced from alginic acid as well as the high standardised production of 2PE occurring during the fermentation of the hydrolysates produced from alginic acid.
4. Conclusions
This work analyses and compares the reactivity of cellulose, hemicellulose and alginic acid when subjected to microwave-assisted hydrothermal treatment for the production of fermentable liquids. The carbohydrates reactivity was evaluated at different operating conditions and the fermentability of the liquids was experimentally assessed using the oleaginous yeast Metschnikowia pulcherrima. The most important conclusions are summarised as follows.1. When subjected to the same operating conditions, the reactivity of the carbohydrates increased as follows: cellulose < hemicellulose < alginic acid. The overall conversion and liquid and gas yields were not affected by the conditions for cellulose. Conversely the temperature and reaction time had a very important influence during the hydrothermal treatment of hemicellulose or alginic acid.
2. The operating conditions did not influence the chemical composition of the hydrolysates produced from hemicellulose and a high concentration of DP > 6 oligosaccharides was obtained in all the experiments. Conversely, the hydrolysates produced from cellulose and alginic acid were influenced by the operating conditions. The former contained high proportions of oligosaccharides and saccharides, the latter comprising high proportions of water-soluble DP > 6 oligomers/oligosaccharides and fermentation inhibitors.
3. The hydrolysates produced from cellulose provided the best results in terms of standardised yeast biomass growth, with lower inhibitors and more accessible oligosaccharides. This did not translate into higher 2PE yields though, with hemicellulose and alginic acid being both suitable materials for the production of this high value compound.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research has been funded by the Industrial Biotechnology Catalyst (Innovate UK, BBSRC, EPSRC) to support the translation, development and commercialisation of innovative Industrial Biotechnology processes (EP/N013522/1). EPSRC for research grant number EP/K014773/1. The authors would like to thank Dr Hannah Briers for her outstanding analytical work and useful inputs during the chemical analysis of the hydrolysates.References
- D. A. Bulushev and J. R. H. Ross, Catal. Today, 2011, 171, 1–13 CrossRef.
- J. C. Escobar, E. S. Lora, O. J. Venturini, E. E. Yáñez, E. F. Castillo and O. Almazan, Renewable Sustainable Energy Rev., 2009, 13, 1275–1287 CrossRef.
- D. Ayhan, Energy Convers. Manage., 2008, 49, 2106–2116 CrossRef.
- J. M. Prado, D. Lachos-Perez, T. Forster-Carneiro and M. A. Rostagno, Food Bioprod. Process., 2016, 98, 95–123 CrossRef.
- P. Alvira, E. Tomas-Pejo, M. Ballesteros and M. J. Negro, Bioresour. Technol., 2010, 101, 4851–4861 CrossRef PubMed.
- Y.-B. Huang and Y. Fu, Green Chem., 2013, 15, 1095 RSC.
- P. Langan, S. Gnanakaran, K. D. Rector, N. Pawley, D. T. Fox, D. W. Cho and K. E. Hammel, Energy Environ. Sci., 2011, 4, 3820 RSC.
- H. Tadesse and R. Luque, Energy Environ. Sci., 2011, 4, 3913 RSC.
- Y.-F. Huang, P.-T. Chiueh and S.-L. Lo, Sustainable Environ. Res., 2016, 26, 103–109 CrossRef.
- S. S. Toor, L. Rosendahl and A. Rudolf, Energy, 2011, 36, 2328–2342 CrossRef.
- W. Jeon, C. Ban, J. E. Kim, H. C. Woo and D. H. Kim, J. Mol. Catal. A: Chem., 2016, 423, 264–269 CrossRef.
- W. Jeon, C. Ban, G. Park, H. C. Woo and D. H. Kim, Catal. Today, 2016, 265, 154–162 CrossRef.
- T. Rogalinski, K. Liu, T. Albrecht and G. Brunner, J. Supercrit. Fluids, 2008, 46, 335–341 CrossRef.
- M. Sasaki, Z. Fang, Y. Fukushima, T. Adschiri and K. Arai, Ind. Eng. Chem. Res., 2000, 39, 2883–2890 CrossRef.
- E. Kamio, H. Sato, S. Takahashi, H. Noda, C. Fukuhara and T. Okamura, J. Mater. Sci., 2007, 43, 2179–2188 CrossRef.
- W. S. L. Mok and M. J. Antal, Ind. Eng. Chem. Res., 1992, 31, 1157–1161 CrossRef.
- V. L. Budarin, J. H. Clark, B. A. Lanigan, P. Shuttleworth and D. J. Macquarrie, Bioresour. Technol., 2010, 101, 3776–3779 CrossRef PubMed.
- V. L. Budarin, P. S. Shuttleworth, J. R. Dodson, A. J. Hunt, B. Lanigan, R. Marriott, K. J. Milkowski, A. J. Wilson, S. W. Breeden, J. Fan, E. H. K. Sin and J. H. Clark, Energy Environ. Sci., 2011, 4, 471–479 RSC.
- T. Li, J. Remón, P. S. Shuttleworth, Z. Jiang, J. Fan, J. H. Clark and V. L. Budarin, Energy Convers. Manage., 2017, 144, 104–113 CrossRef.
- A. Mamaeva, A. Tahmasebi, L. Tian and J. Yu, Bioresour. Technol., 2016, 211, 382–389 CrossRef PubMed.
- R. Omar and J. P. Robinson, J. Anal. Appl. Pyrolysis, 2014, 105, 131–142 CrossRef.
- D. Rosso, J. Fan, E. Montoneri, M. Negre, J. Clark and D. Mainero, Green Chem., 2015, 17, 3424–3435 RSC.
- Y. Q. Wan, P. Chen, B. Zhang, C. Y. Yang, Y. H. Liu, X. Y. Lin and R. Ruan, J. Anal. Appl. Pyrolysis, 2009, 86, 161–167 CrossRef.
- Z. Zhang, D. J. Macquarrie, M. De bruyn, V. L. Budarin, A. J. Hunt, M. J. Gronnow, J. Fan, P. S. Shuttleworth, J. H. Clark and A. S. Matharu, Green Chem., 2015, 17, 260–270 RSC.
- E. M. de Melo, J. H. Clark and A. S. Matharu, Green Chem., 2017, 19, 3408–3417 RSC.
- J. Fan, M. De bruyn, V. L. Budarin, M. J. Gronnow, P. S. Shuttleworth, S. Breeden, D. J. Macquarrie and J. H. Clark, J. Am. Chem. Soc., 2013, 135, 11728–11731 CrossRef PubMed.
- J. Fan, M. De bruyn, Z. Zhu, V. Budarin, M. Gronnow, L. D. Gomez, D. Macquarrie and J. Clark, Chem. Eng. Process., 2013, 71, 37–42 CrossRef.
- F. Li, L. Liu, Y. An, W. He, N. J. Themelis and G. Li, J. Cleaner Prod., 2016, 112, 1049–1054 CrossRef.
- N. Mahmood, Z. Yuan, J. Schmidt and C. Xu, Renewable Sustainable Energy Rev., 2016, 60, 317–329 CrossRef.
- R. Singh, B. B. Krishna, G. Mishra, J. Kumar and T. Bhaskar, Renewable Energy, 2016, 98, 226–237 CrossRef.
- M. F. Li, S. N. Sun, F. Xu and R. C. Sun, Food Chem., 2012, 134, 1392–1398 CrossRef PubMed.
- M. F. Li, S. N. Sun, F. Xu and R. C. Sun, Food Chem., 2012, 134, 1392–1398 CrossRef PubMed.
- T. Li, J. Remón, Z. Jiang, V. L. Budarin and J. H. Clark, Energy Convers. Manage., 2018, 155, 147–160 CrossRef.
- F. Monteil-Rivera, G. H. Huang, L. Paquet, S. Deschamps, C. Beaulieu and J. Hawari, Bioresour. Technol., 2012, 104, 775–782 CrossRef PubMed.
- L. Zhou, V. Budarin, J. Fan, R. Sloan and D. Macquarrie, ACS Sustainable Chem. Eng., 2017, 5, 3768–3774 CrossRef.
- L. Zhou, F. Santomauro, J. Fan, D. Macquarrie, J. Clark, C. J. Chuck and V. Budarin, Faraday Discuss., 2017, 202, 351–370 RSC.
- S. Zhou, L. Liu, B. Wang, F. Xu and R. Sun, Process Biochem., 2012, 47, 1799–1806 CrossRef.
- L. Zoia, M. Orlandi and D. S. Argyropoulos, J. Agric. Food Chem., 2008, 56, 10115–10122 CrossRef PubMed.
- M. De bruyn, J. Fan, V. L. Budarin, D. J. Macquarrie, L. D. Gomez, R. Simister, T. J. Farmer, W. D. Raverty, S. J. McQueen-Mason and J. H. Clark, Energy Environ. Sci., 2016, 9, 2571–2574 RSC.
- J. Remón, A. S. Matharu and J. H. Clark, Energy Convers. Manage., 2018, 165, 634–648 CrossRef.
- N. Mosier, C. Wyman, B. Dale, R. Elander, Y. Y. Lee, M. Holtzapple and M. Ladisch, Bioresour. Technol., 2005, 96, 673–686 CrossRef PubMed.
- Z. Jiang, J. Yi, J. Li, T. He and C. Hu, ChemSusChem, 2015, 8, 1901–1907 CrossRef PubMed.
- Z. Jiang, V. L. Budarin, J. Fan, J. Remón, T. Li, C. Hu and J. H. Clark, ACS Sustainable Chem. Eng., 2018, 6(3), 4098–4104 CrossRef.
- Y. Wang, F. Delbecq, R. S. Varma and C. Len, Mol. Catal., 2018, 445, 73–79 CrossRef.
- T. Chantasuban, F. Santomauro, D. Gore-Lloyd, S. Parsons, D. Henk, R. J. Scott and C. Chuck, J. Chem. Technol. Biotechnol., 2018, 93(8), 2118–2130 CrossRef PubMed.
- J. R. Busemeyer and A. Diederich, in Neuroeconomics, ed. P. W. Glimcher and E. Fehr, Academic Press, San Diego, 2nd edn, 2014, pp. 49–61, DOI:10.1016/B978-0-12-416008-8.00004-8.
- E. A. Mohammed, C. Naugler and B. H. Far, in Emerging Trends in Computational Biology, Bioinformatics, and Systems Biology, ed. Q. N. Tran and H. Arabnia, Morgan Kaufmann, Boston, 2015, pp. 577–602, DOI:10.1016/B978-0-12-802508-6.00032-6.
- J. Remón, F. Broust, G. Volle, L. García and J. Arauzo, Int. J. Hydrogen Energy, 2015, 40, 5593–5608 CrossRef.
- C. Briens, J. Piskorz and F. Berruti, Int. J. Chem. React. Eng., 2008, 6, 51 Search PubMed.
- I. Egües, M. G. Alriols, Z. Herseczki, G. Marton and J. Labidi, J. Ind. Eng. Chem., 2010, 16, 293–298 CrossRef.
- H. Pińkowska, P. Wolak and E. Oliveros, Biomass Bioenergy, 2014, 64, 50–61 CrossRef.
- J. Remón, L. García and J. Arauzo, Fuel Process. Technol., 2016, 154, 66–81 CrossRef.
- J. Remón, M. Laseca, L. García and J. Arauzo, Energy Convers. Manage., 2016, 114, 122–141 CrossRef.
- J. Remón, J. Ruiz, M. Oliva, L. García and J. Arauzo, Energy Convers. Manage., 2016, 124, 453–469 CrossRef.
- J. N. Chheda and J. A. Dumesic, Catal. Today, 2007, 123, 59–70 CrossRef.
- G. W. Huber and J. A. Dumesic, Catal. Today, 2006, 111, 119–132 CrossRef.
- A. V. Kirilin, A. V. Tokarev, L. M. Kustov, T. Salmi, J. P. Mikkola and D. Y. Murzin, Appl. Catal., A, 2012, 435–436, 172–180 CrossRef.
- D. W. Rackemann, J. P. Bartley and W. O. S. Doherty, Ind. Crops Prod., 2014, 52, 46–57 CrossRef.
- M. J. Taylor, L. J. Durndell, M. A. Isaacs, C. M. A. Parlett, K. Wilson, A. F. Lee and G. Kyriakou, Appl. Catal., B, 2016, 180, 580–585 CrossRef.
- J. Tuteja, S. Nishimura and K. Ebitani, Bull. Chem. Soc. Jpn., 2012, 85, 275–281 CrossRef.
- K. Yan, G. Wu, T. Lafleur and C. Jarvis, Renewable Sustainable Energy Rev., 2014, 38, 663–676 CrossRef.
- Y. Fan, Y. Cai, X. Li, L. Jiao, J. Xia and X. Deng, Energy Convers. Manage., 2017, 138, 106–118 CrossRef.
- T. Taubner, M. Marounek and A. Synytsya, Int. J. Biol. Macromol., 2017, 103, 202–207 CrossRef PubMed.
- K. Marta, P. S. Belton, R. H. Wilson, H. Ján and E. Anna, J. Sci. Food Agric., 1998, 77, 38–44 CrossRef.
- Z. Cheng, J. L. Everhart, G. Tsilomelekis, V. Nikolakis, B. Saha and D. G. Vlachos, Green Chem., 2018, 20, 997–1006 RSC.
- M.-Z. Ding, X. Wang, W. Liu, J.-S. Cheng, Y. Yang and Y.-J. Yuan, PLoS One, 2012, 7, e43474 CrossRef PubMed.
- F. Whiffin, F. Santomauro and C. J. Chuck, Biofuels, Bioprod. Biorefin., 2016, 10, 316–334 CrossRef.
- X. Yu, Y. Zheng, K. M. Dorgan and S. Chen, Bioresour. Technol., 2011, 102, 6134–6140 CrossRef PubMed.
- F. Santamauro, F. M. Whiffin, R. J. Scott and C. J. Chuck, Biotechnol. Biofuels, 2014, 7, 34 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8gc02182a |
| This journal is © The Royal Society of Chemistry 2018 |