Carbene-catalyzed aerobic oxidation of isoquinolinium salts: efficient synthesis of isoquinolinones†
Abstract
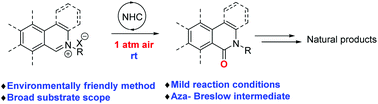
A mild and environmentally friendly carbene-catalyzed aerobic oxidation of isoquinolinium salts was successfully realized. Accordingly, a diverse set of isoquinolinones and phenanthridinones was efficiently prepared in good to excellent yields. The mechanistic study indicates that the formation of an aza-Breslow intermediate is the crucial step in this transformation. This reaction features ambient air as the sole oxidant and oxygen source, a broad substrate scope, and excellent functional-group tolerance and proceeds under mild reaction conditions. Furthermore, a highly efficient synthesis of bioactive molecules and natural products including N-methylcrinasiadine, N-isopentylcrinasiadine, N-phenethylcrinasiadine, isoindolo[2,1-b]isoquinolin-5(7H)-one, PJ-34, rac-Gusanlung D, rosettacin, 8-oxopseudopalmatine and ilicifoline B was accomplished.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...