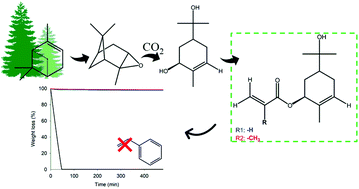
Sobrerol acrylate (SobAcr) and sobrerol methacrylate (SobMet) were synthesized for the first time through the functionalization of sobrerol with double bonds. Sobrerol was obtained by a green route that involved the hydration of α-pinene oxide in the presence of CO2. As unsaturated monomers (UMs) with rigid structures and extremely low volatilities, these new monomers are interesting alternatives for styrene (Sty) replacement in unsaturated polyester resin (UPR) formulations. The impact of the substitution of Sty by the new Sob-based monomers was studied using three different unsaturated polyesters (UPs): one commercially available and two fully bio-based that are synthesized in this work. The Sob-based monomers were able to fully dissolve the three UPs. Although UPRs with SobAcr showed higher thermal stability than SobMet-based materials, SobMet-based formulations presented higher glass transition temperatures (Tg) and storage moduli (E′). Overall, both Sob-based monomers exhibited promising properties to be used as low-volatile, bio-based effective Sty substitutes, and by that means mitigate a serious environmental issue concerning human exposure to toxic, volatile organic compounds (VOCs).