Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbon nitride creates thioamides in high yields by the photocatalytic Kindler reaction†
B.
Kurpil
 ,
B.
Kumru
,
T.
Heil
,
M.
Antonietti
,
B.
Kumru
,
T.
Heil
,
M.
Antonietti
 and
A.
Savateev
and
A.
Savateev
 *
*
Max-Planck Institute of Colloids and Interfaces, Department of Colloid Chemistry, Research Campus Golm, 14424 Potsdam, Germany. E-mail: oleksandr.savatieiev@mpikg.mpg.de
First published on 6th February 2018
Abstract
Potassium poly(heptazine imide), a carbon nitride based photocatalyst, effectively promotes the Kindler reaction of thioamide bond formation using amines and elemental sulfur as building blocks under visible light irradiation. The feasibility of the developed methodology was confirmed using 14 different primary and secondary amines, including substituted benzylamines and heterocyclic and aliphatic methylamines, which were successfully converted into thioamides with 68–92% isolated yields.
In recent years, usage of visible light energy as a driving force for organic synthesis has gained significant attention.1,2 A large body of studies has been published, where different organic dyes3–6 or metal complexes7–9 are used as photocatalysts. These catalysts have already proven to be very efficient for a broad range of photocatalytic reactions. Nevertheless, the increasing applicability puts new requests on the photocatalyst price, durability, efficiency and reusability and motivates scientists to search for new materials that would meet the aforementioned requirements. In this view, graphitic carbon nitride could complement the toolbox of well-acknowledged photocatalysts,10–13 like [Ir(ppy)2(dtb-bpy)]PF6,14 [Ru(bpy)3]15etc. The use of carbon nitride in addition expands the spectrum of organic photoreactions and enables reaction conditions under which metal based photocatalysts are not stable, e.g. those involving organophosphorus or organosulfur reagents. Carbon nitride as a heterogeneous, metal free system is known to be stable against all those and also NOx, CO, singlet oxygen and many others.
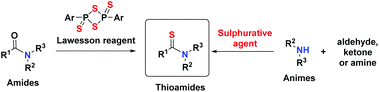
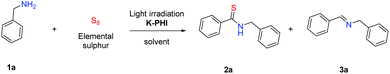
Potassium poly(heptazine imide), hereafter K-PHI, is a recent addition to the family of carbon nitride like materials (Fig. 1).16 Different from polymeric graphitic carbon nitride, it has an ionic nature and can, for instance, reversibly exchange cations in salt solution and form composites with MOFs.17,18 The main feature important for photocatalysis is however its highly positive valence band potential (+2.6 eV) and the effective photocharge separation in this system.19 The ultrahigh charge carrier mobility enhances the catalytic activity in the photooxidation process.20 K-PHI has already proved its effectiveness in the metal free water oxidation and oxidation of alcohols accompanied by Hantzsch pyridine synthesis.21,22 Besides, K-PHI is based only on organic mass chemicals, which makes a targeted prize for a scaled up version in the range of a few € per kg possible.
In the current contribution, we intended to reanalyze the problem of photochemical amine oxidation, using elemental sulfur as a sacrificial electron acceptor. This situation mimics in one or the other way the reductive character of Earth's early oceans and atmosphere and favors carbon nitride to be inert against such conditions.
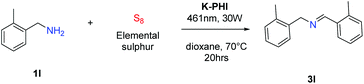
Interestingly, it turned out that K-PHI is a very effective photocatalyst to create thioamides, which often occur in the structure of biologically active compounds,23,24 and also serves as a convenient building block to synthesize complex organic molecules25,26 or in total synthesis.27 Among the existing methods for the synthesis of thioamides the basic methods are: transformation of amides into thioamides using Lawesson's reagent,28,29 and the Kindler thioamide synthesis – a multicomponent reaction between aldehydes,30–33 ketones34 or amines35 and sulfuration reagents (Scheme 1). However, these methods often face the problem of low conversions, harsh reaction conditions, and the usage of less pleasant reagents.
Elemental sulfur on the other hand is omni-available, non-toxic, and low priced, and its utilization as a reagent in thioamide synthesis looks appealing. Following these thoughts, in this work we have developed a new method that furnishes thioamides and even short chain thiopeptides from the corresponding amines and elemental sulfur in high yields and selectivities under visible light irradiation using K-PHI as a photocatalyst.
The photocatalyst, K-PHI, was synthesized according to the described procedure.22 In the first model reaction of thioamide 2a synthesis, benzylamine was used as a reference molecule to optimize the reaction conditions (Scheme 2, Table 1). Thioamide 2a was not formed in the control experiments, without photocatalysts (entry 1) or light irradiation (entry 2). When K-PHI was used as a photocatalyst and the reaction mixture was irradiated with a blue light diode, the highest conversion of benzylamine (99%) and the highest selectivity with respect to thioamide 2a were obtained after 20 hours of running the reaction at 70 °C in dioxane (entries 3 and 7) or after 100 hours at 30 °C (entry 9). The durability of K-PHI was assessed (entries 4 and 5). After the third cycle, conversion remained at 99%, while selectivity toward thioamide had slightly decreased. In general, the structure of K-PHI is stable under the conditions of thioamide synthesis as concluded from the identity of powder X-Ray diffraction (PXRD) patterns and Fourier-transform infrared (FT-IR) spectra of this material before and after photocatalytic experiments (Fig. S2†). When tetrahydrofuran (THF) was used as a solvent (entries 6, 8 and 10) both selectivity and conversion were found to be slightly lower. We attribute these findings to a lower stability of THF compared to dioxane under the given reaction conditions – small quantities of tetrahydrothiophene were detected in the reaction mixture, indicating the sensitivity of THF against cationic attack. A lower selectivity for thioamide (30%) was observed when the reaction was performed in benzene (entry 11). In non-polar solvents in general, the heterogeneous photocatalysts’ particles agglomerate, and only a small fraction of the particles participate in light absorption and subsequent photocatalytic transformations. Indeed, aqueous solvent systems would be ideal, but were not chosen because of low substrate solubility.
| Entry | Solvent | Catalyst | Temp (°C) | Time (h) | Conv. (%) |
2a/3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|---|---|---|---|---|---|---|
| a Reaction conditions: Benzylamine (0.5 mmol), sulphur (1.5 mmol), photocatalyst (20 mg), solvent (2 ml), λ = 461 nm. b Molar ratio between compounds 2a and 3a was determined by 1H NMR. c Without photocatalysts. d Without light irradiation. e Recycled K-PHI (second run). f Recycled K-PHI (third run). g 10 mg of photocatalyst. | ||||||
| 1c | Dioxane | — | 70 | 20 | 10 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2d | Dioxane | K-PHI | 70 | 20 | 18 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Dioxane | K-PHI | 70 | 20 | 99 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4e | Dioxane | K-PHI | 70 | 20 | 99 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5f | Dioxane | K-PHI | 70 | 20 | 99 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | THF | K-PHI | 70 | 20 | 99 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7g | Dioxane | K-PHI | 70 | 20 | 99 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8g | THF | K-PHI | 70 | 20 | 70 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9g | Dioxane | K-PHI | 30 | 100 | 99 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10g | THF | K-PHI | 30 | 100 | 97 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 11 | Benzene | K-PHI | 70 | 20 | 99 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 12 | Methanol | K-PHI | 70 | 20 | 80 | — |
| 13 | Dioxane | mpg-CN | 70 | 20 | 51 | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | Dioxane | Na-PHI | 70 | 20 | 64 | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Methanol (entry 12) as a redox active solvent under the reaction conditions is oxidized to formaldehyde that reacts with benzyl amine giving a mixture of unidentified products as evidenced by 1H NMR. In order to compare the photocatalytic activity of K-PHI with other carbon nitride materials, mesoporous graphitic carbon nitride (mpg-CN) was tested under the optimized conditions (entry 13). Significantly lower conversion (51%) and selectivities toward thioamide 2a were observed. It may be explained by the existence of the competitive pathways in which an intermediate imine could potentially participate and which are much better promoted by mpg-CN. Interestingly, the activity of Na-PHI under identical conditions was lower than that of K-PHI (entry 14). The main reason apparently lies in morphological differences of these two materials. The rod-like morphology with a pronounced stacking of poly(heptazine imide) layers in K-PHI facilitates the transport of the photogenerated charge carriers between the layers better compared to the plate-like morphology of Na-PHI.16 Also more selective absorption of reagents on the surface of K-PHI compared to Na-PHI cannot be excluded.
To analyse the potential width of this reaction, we expanded the substrate scope onto substituted benzylamines and heterocyclic and aliphatic amines. The results with isolated yields are summarized in Scheme 3. Thus, the model thioamide 2a was isolated with 90% yield. Under the optimized reaction conditions substituted benzylamines gave corresponding thioamides 2b–d in excellent yields. At the same time 4-aminobenzylamine gave corresponding thioamide 2e with slightly lower yield (76%). This could be due to the presence of the aromatic amino group that could also be oxidized by K-PHI. Notably, 2- and 3-picolylamines and also 2-aminomethylfuran gave thioamides 2f,g,i also with high yields. In the case of 4-picolylamine, the corresponding thioamide 2h was obtained along with a small amount of 4-cyanopyridine as proved by GC-MS. Apparently, the latter is the product of oxidative dehydrogenation of the starting amine. Because of the high sensitivity of the intermediates of aliphatic thioamides 2j,k, we were unable to isolate these compounds when the reaction was performed at 70 °C. However, due to high activity, K-PHI enables the selective oxidation of organic substrates even at room temperature – thioamides 2j,k were obtained in high yields. At those temperatures, the reaction time was extended, and tert-butanol was used as a solvent. The value of K-PHI as a photocatalyst for synthetic organic chemistry can be illustrated by thioamides 2b,e,g,h,j,k which are not described in the literature. These molecules quite likely could not be synthesized using the traditional methods listed at the beginning of this report. Nevertheless, K-PHI synthesizes the aforementioned molecules in high yields.
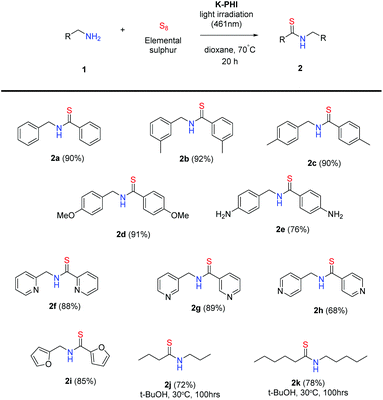 | ||
| Scheme 3 Photocatalytic preparation of thioamides from aryl- and hetarylmethylamines and aliphatic amines. The isolated yields are given in brackets. | ||
Interestingly, the reactivity of 2-methylbenzylamine 1l differs drastically from the rest of the analysed amines (Scheme 4). Under the standard reaction conditions it does not give any thioamides, and only imine 3l was isolated as the sole product. This is possible due to the steric hindrance created by the methyl group near the reactive centre.
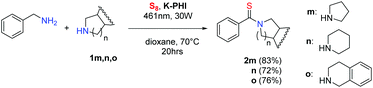
The developed reaction of photocatalytic thioamide synthesis was finally expanded onto the synthesis of non-symmetric thioamides, those obtained from two different amines (Scheme 5). A threefold excess of amines 1m–oversus benzyl amine allowed for thioamides 2m–o synthesis in 72–83% isolated yields.
Finally, the feasibility of the developed method was applied in a short chain thiopeptide synthesis. p-Xylylenediamine and m-xylylenediamine were selected as the substrates. The chain extension is apparently terminated at the trimer and dimer steps in the case of p- and m-xylylenediamine, respectively, as evidenced by size-exclusion chromatography (SEC) most probably only because of the low solubility of these thiopeptides (Fig. S3†). We therefore expect higher molecular weights of thiopeptides for aliphatic amines with their higher solubility. This, however, is a subject of ongoing research.
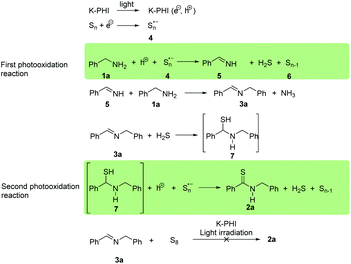
A possible mechanism scheme of the thioamide photocatalytic synthesis using the example of thioamide 2a is illustrated in Scheme 6. In the initial step, a hole (h+) and electron (e−) pair is generated upon K-PHI irradiation with visible light. Similarly to oxygen,36 elemental sulfur is reduced by the photogenerated electron (e−) affording the polysulfide radical anion 4. Carbon nitride photocatalyzed oxidation of amines into the corresponding imines using O2 as a sacrificial electron/proton acceptor was reported in the literature earlier.37 In all these schemes, the oxidation of benzylamine 1a by the photogenerated hole in the presence of radical anion 4 yields imine 5. Polymers or oligomeric sulfur chains 6 can disproportionate under the evolution of H2S. The presence of hydrogen sulfide was confirmed as Ag2S by passing the gases evolved during the reaction through the AgNO3 solution. The formation of imine 3a from the benzylamine 1a and imine 5 seems to occur according to the previously reported mechanism.37 The addition of H2S to the imine's 3a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond produces an intermediate α-aminothiol 7, as was postulated earlier.30,31 Our results agree with these data. Thus, the independently synthesized imine 3a did not give thioamide 2a under identical photocatalytic conditions. Another observation taken as evidence supporting this mechanism is that 2-methylbenzylamine 1l under the photocatalytic conditions gives only imine 3l probably because of the steric hindrance of the methyl groups that effectively shield C
N bond produces an intermediate α-aminothiol 7, as was postulated earlier.30,31 Our results agree with these data. Thus, the independently synthesized imine 3a did not give thioamide 2a under identical photocatalytic conditions. Another observation taken as evidence supporting this mechanism is that 2-methylbenzylamine 1l under the photocatalytic conditions gives only imine 3l probably because of the steric hindrance of the methyl groups that effectively shield C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond against H2S addition (Scheme 5). Notably, the yield of thioamide 2a decreases significantly when the reaction is accomplished in an open reactor enabling H2S to escape from the reaction medium. The oxidation of α-aminothiol 7 to thioamide 2a using chemical oxidants such as molecular iodine or K2S2O8 was reported before.30,31 In the present case, the oxidation of α-aminothiol 7 is accomplished by a further photogenerated hole (h+) in the presence of polysulfide radical anions. This one-pot, consecutive double photocatalytic oxidation with K-PHI thereby is efficiently utilized in the synthesis of complex organic molecules, such as thioamides and even short chain thiopeptides, from small molecules under very mild conditions.
N bond against H2S addition (Scheme 5). Notably, the yield of thioamide 2a decreases significantly when the reaction is accomplished in an open reactor enabling H2S to escape from the reaction medium. The oxidation of α-aminothiol 7 to thioamide 2a using chemical oxidants such as molecular iodine or K2S2O8 was reported before.30,31 In the present case, the oxidation of α-aminothiol 7 is accomplished by a further photogenerated hole (h+) in the presence of polysulfide radical anions. This one-pot, consecutive double photocatalytic oxidation with K-PHI thereby is efficiently utilized in the synthesis of complex organic molecules, such as thioamides and even short chain thiopeptides, from small molecules under very mild conditions.
In summary, potassium poly(heptazine imide) showed high efficacy in a photocatalytic, metal-free thioamide bond formation. In the developed method different amines and elemental sulfur were used as reagents while visible light acted as a driving force to accomplish the reaction. Using this method, different thioamides, also previously not known species, bearing alkyl, aryl and hetaryl substituents were isolated in good to excellent yields. Limitations of this reaction were shown as well. The developed method also opens new horizons in biochemistry for example, photocatalytic terminal NH-functionalization of peptides. On the other hand, thiopeptides can be easily converted into peptides under aqueous conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Deutsche Forschungsgemeinschaft for the financial support (DFG-An 156 13-1). Open Access funding provided by the Max Planck Society.Notes and references
- D. Friedmann, A. Hakki, H. Kim, W. Choi and D. Bahnemann, Green Chem., 2016, 18, 5391–5411 RSC
.
- J. Chen, J. Cen, X. Xu and X. Li, Catal. Sci. Technol., 2016, 6, 349–362 CAS
.
- J. H. Park, K. C. Ko, E. Kim, N. Park, J. H. Ko, D. H. Ryu, T. K. Ahn, J. Y. Lee and S. U. Son, Org. Lett., 2012, 14, 5502–5505 CrossRef CAS PubMed
.
- T. Hering, T. Slanina, A. Hancock, U. Wille and B. König, Chem. Commun., 2015, 51, 6568–6571 RSC
.
- D. J. Wilger, N. J. Gesmundo and D. A. Nicewicz, Chem. Sci., 2013, 4, 3160–3165 RSC
.
- A. J. Perkowski and D. A. Nicewicz, J. Am. Chem. Soc., 2013, 135, 10334–10337 CrossRef CAS PubMed
.
- M. Rueping, C. Vila, A. Szadkowska, R. M. Koenigs and J. Fronert, ACS Catal., 2012, 2, 2810–2815 CrossRef CAS
.
- Y.-H. Li, X.-L. Liu, Z.-T. Yu, Z.-S. Li, S.-C. Yan, G.-H. Chen and Z.-G. Zou, Dalton Trans., 2016, 45, 12400–12408 RSC
.
- Z. Xie, C. Wang, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 2056–2059 CrossRef CAS PubMed
.
- G. Zhang, Z.-A. Lan and X. Wang, Chem. Sci., 2017, 8, 5261–5274 RSC
.
- A. Savateev, Z. P. Chen and D. Dontsova, RSC Adv., 2016, 6, 2910–2913 RSC
.
- A. Savateev, S. Pronkin, J. D. Epping, M. G. Willinger, M. Antonietti and D. Dontsova, J. Mater. Chem. A, 2017, 5, 8394–8401 CAS
.
- G. Zhang, A. Savateev, Y. Zhao, L. Li and M. Antonietti, J. Mater. Chem. A, 2017, 5, 12723–12728 CAS
.
- P. Kohls, D. Jadhav, G. Pandey and O. Reiser, Org. Lett., 2012, 14, 672–675 CrossRef CAS PubMed
.
- D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed
.
- Z. Chen, A. Savateev, S. Pronkin, V. Papaefthimiou, C. Wolff, M. G. Willinger, E. Willinger, D. Neher, M. Antonietti and D. Dontsova, Adv. Mater., 2017, 29, 1700555 CrossRef PubMed
.
- A. Savateev, S. Pronkin, M. Willinger, M. Antonietti and D. Dontsova, Chem. – Asian J., 2017, 12, 1517–1522 CrossRef CAS PubMed
.
- N. A. Rodríguez, A. Savateev, M. A. Grela and D. Dontsova, ACS Appl. Mater. Interfaces, 2017, 9, 22941–22949 Search PubMed
.
- A. Savateev, S. Pronkin, J. D. Epping, M. G. Willinger, C. Wolff, D. Neher, M. Antonietti and D. Dontsova, ChemCatChem, 2017, 9, 167–174 CrossRef CAS
.
- G. Zhang, G. Li, Z.-a. Lan, L. Lin, A. Savateev, T. Heil, S. Zafeiratos, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2017, 56, 13445–13449 CrossRef CAS PubMed
.
- B. Kurpil, A. Savateev, V. Papaefthimiou, S. Zafeiratos, T. Heila, S. Özenler, D. Dontsova and M. Antonietti, Appl. Catal., B, 2017, 217, 622–628 CrossRef CAS
.
- A. Savateev, D. Dontsova, B. Kurpil and M. Antonietti, J. Catal., 2017, 350, 203–211 CrossRef CAS
.
- L. Lankiewicz, C. Y. Bowers, G. A. Reynolds, V. Labroo, L. A. Cohen, S. Vonhof, A.-L. Sirén and A. F. Spatola, Biochem. Biophys. Res. Commun., 1992, 184, 359–366 CrossRef CAS PubMed
.
- D. B. Sherman, A. F. Spatola, W. S. Wire, T. F. Burks, T. M.-D. Nguyen and P. W. Schiller, Biochem. Biophys. Res. Commun., 1989, 162, 1126–1132 CrossRef CAS PubMed
.
- P. S. Chaudhari, S. P. Pathare and K. G. Akamanchi, J. Org. Chem., 2012, 77, 3716–3723 CrossRef CAS PubMed
.
- F.-Q. Liu, Y.-Q. Qin, L.-Z. Xu, L.-D. Lu, X.-J. Yang and X. Wang, Chin. J. Chem., 2005, 23, 881–884 CrossRef CAS
.
- P. Gopinath, T. Watanabe and M. Shibasaki, J. Org. Chem., 2012, 77, 9260–9267 CrossRef CAS PubMed
.
- M. P. Cava and M. I. Levinson, Tetrahedron, 1985, 41, 5061–5087 CrossRef CAS
.
- Z. Kaleta, B. T. Makowski, T. Soós and R. Dembinski, Org. Lett., 2006, 8, 1625–1628 CrossRef CAS PubMed
.
- H.-Z. Li, W.-J. Xue, G.-D. Yin and A.-X. Wu, Tetrahedron Lett., 2015, 56, 5843–5846 CrossRef CAS
.
- J. Wei, Y. Li and X. Jiang, Org. Lett., 2016, 18, 340–343 CrossRef CAS PubMed
.
- O. I. Zbruyev, N. Stiasni and C. O. Kappe, J. Comb. Chem., 2003, 5, 145–148 CrossRef CAS PubMed
.
- S. P. Pathare, P. S. Chaudhari and K. G. Akamanchi, Appl. Catal., A, 2012, 425–426, 125–129 CrossRef CAS
.
- K. Okamoto, T. Yamamoto and T. Kanbara, Synlett, 2007, 2687–2690 CAS
.
- T. B. Nguyen, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2012, 14, 4274–4277 CrossRef CAS PubMed
.
- K. H. Wujcik, D. R. Wang, A. Raghunathan, M. Drake, T. A. Pascal, D. Prendergast and N. P. Balsara, J. Phys. Chem. C, 2016, 120, 18403–18410 CAS
.
- F. Su, S. C. Mathew, L. Möhlmann, M. Antonietti, X. Wang and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657–660 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7gc03734a |
| This journal is © The Royal Society of Chemistry 2018 |