Correlation of drug-induced and drug-related ultra-high performance liquid chromatography-mass spectrometry serum metabolomic profiles yields discovery of effective constituents of Sini decoction against myocardial ischemia in rats†
Abstract
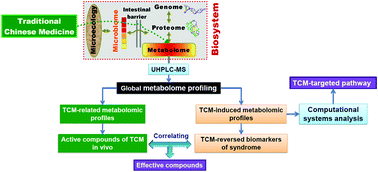
Screening active constituents of traditional Chinese medicines (TCMs) is vital for lead compound discovery. Sini decoction (SND) is a well-known TCM formula for relieving myocardial ischemia (MI) in clinic. Due to complex nature, the effective compounds of SND are still unknown. In this study, a novel “system to system” strategy based on the correlation of drug-related and drug-induced ultra-high performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry (UHPLC-Q-TOFMS) serum metabolomic profiles was developed to discover bioactive compounds of SND against isoproterenol-induced MI. Thirteen SND-induced metabolites and 19 SND-related metabolites were identified by UHPLC-Q-TOFMS coupled with S-plot and SUS-plot of orthogonal projection to latent structure-discriminant analysis (OPLS-DA) models, respectively. Canonical correlation analysis between the SND-induced and SND-related metabolites revealed that 12 compounds had strongly correlated relationship with the protective effect of SND on MI, and these compounds include isotalatizidine, songorine, fuziline, neoline, talatizamine, 14-acetyltalatizamine, liquiritigenin, benzoylmesaconitine, isoliquiritin, benzoylaconitne, benzoylhypaconitine and 6-gingerol. Combination functional enrichment analysis and network topology analysis revealed that the targeted metabolic pathways of these correlated compounds were involved in valine, leucine and isoleucine biosyntheses, tryptophan metabolism, glycerophospholipid metabolism and sphingolipid metabolism. The results demonstrated that the “system to system” strategy may be a high-throughput method to discover potentially effective compounds from TCMs.

 Please wait while we load your content...
Please wait while we load your content...