Ginsenoside Rg5 induces apoptosis and autophagy via the inhibition of the PI3K/Akt pathway against breast cancer in a mouse model
Abstract
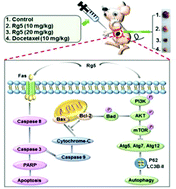
Breast cancer is the most frequently diagnosed cancer and has become the main cause of cancer-related death among women worldwide. Traditional chemotherapy for breast cancer has serious side effects for patients, such as the first-line drug docetaxel. Ginsenoside Rg5, a rare ginsenoside and the main ingredient extracted from fine black ginseng, has been proved to have anti-breast cancer efficacy in vitro. Here, the in vivo anti-breast cancer efficacy, side effects and potential molecular mechanisms of Rg5 were investigated on a BALB/c nude mouse model of human breast cancer. The tumor growth inhibition rate of high dose Rg5 (20 mg kg−1) was 71.4 ± 9.4%, similar to that of the positive control docetaxel (72.0 ± 9.1%). Compared to docetaxel, Rg5 showed fewer side effects in the treatment of breast cancer. Treatment with Rg5 induced apoptosis and autophagy in breast cancer tissues. Rg5 was proved to induce caspase-dependent apoptosis via the activation of the extrinsic death receptor and intrinsic mitochondrial signaling pathways. The autophagy induction was related to the formation of an autophagosome and accumulation of LC3BII, P62 and critical Atg proteins. Further studies showed that Rg5 in a dose-dependent manner induced apoptosis and autophagy through the inhibition of the PI3K/Akt signaling pathway as indicated by the reduced phosphorylation level of PI3K and Akt. Taken together, Rg5 could be a novel and promising clinical antitumor drug targeting breast cancer.


 Please wait while we load your content...
Please wait while we load your content...