Aspalathus linearis (Rooibos) – a functional food targeting cardiovascular disease
Abstract
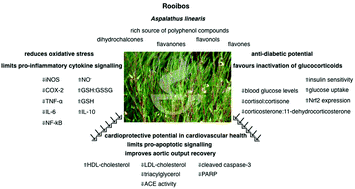
Increasing consumer bias toward natural products and the considerable wealth of indigenous knowledge has precipitated an upturn in market-driven research into potentially beneficial medicinal plants. In this context, Aspalathus linearis (Rooibos) has been identified to be a promising candidate which may impact cardiovascular disease (CVD), which is one of the most widely studied chronic diseases of modern times. Despite these efforts, ischemic heart disease remains the number one cause of mortality globally. Apart from genetic predisposition and other aetiological mechanisms specific to particular types of CVD, co-factors from interlinked systems contribute significantly to disease development and the severity of its clinical manifestation. The bioactivity of Rooibos is directed towards multiple therapeutic targets. Experimental data to date include antioxidant, anti-inflammatory and anti-diabetic effects, as well as modulatory effects in terms of the immune system, adrenal steroidogenesis and lipid metabolism. This review integrates relevant literature on the therapeutic potential of Rooibos in the context of CVD, which is currently the most common of non-communicable diseases. The therapeutic value of whole plant extracts versus isolated active ingredients are addressed, together with the potential for overdose or herb–drug interaction. The body of research undertaken to date clearly underlines the benefits of Rooibos as both preventative and complementary therapeutic functional food in the context of CVD.


 Please wait while we load your content...
Please wait while we load your content...