Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of a low and a high dietary LA/ALA ratio on long-chain PUFA concentrations in red blood cells†
Theresa
Greupner
 a,
Laura
Kutzner
b,
Svenja
Pagenkopf
a,
Heike
Kohrs
a,
Andreas
Hahn
a,
Nils Helge
Schebb
bc and
Jan Philipp
Schuchardt
*a
a,
Laura
Kutzner
b,
Svenja
Pagenkopf
a,
Heike
Kohrs
a,
Andreas
Hahn
a,
Nils Helge
Schebb
bc and
Jan Philipp
Schuchardt
*a
aInstitute of Food Science and Human Nutrition, Leibniz University Hannover, Germany. E-mail: schuchardt@nutrition.uni-hannover.de; Fax: +49 (0)511-7625729; Tel: +49 (0)511-762 2987
bInstitute for Food Toxicology, University of Veterinary Medicine Hannover, Germany
cChair of Food Chemistry, Faculty of Mathematics and Natural Sciences, University of Wuppertal, Germany
First published on 13th August 2018
Abstract
There is a debate about the optimal dietary ratio of the parent n6 fatty acid linoleic acid (LA) and n3 fatty acid alpha-linolenic acid (ALA) to promote an efficient conversion of ALA to EPA and DHA, which have implications for human health. The aim of the present study was to compare the effects of a low-LA/high-ALA (loLA/hiALA) diet with a high-LA/low-ALA (hiLA/loALA) diet on fatty acid concentrations in red blood cells (RBCs). Fifteen omnivore healthy men (mean age 26.1 ± 4.5 years) with a low initial EPA/DHA status (sum (∑) EPA + DHA% of total fatty acids in RBC at baseline: 4.03 ± 0.17) received both diets for two weeks with a nine-week wash-out phase in between. Fatty acid intake of the subjects was tightly controlled. Concentrations [μg mL−1] and relative amounts [% of total fatty acids] of fatty acids in RBCs were analyzed at baseline (day 0), day 7 and 14 by means of GC-FID. The dietary LA/ALA ratios were 0.56 ± 0.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 25.6 ± 2.41
1 and 25.6 ± 2.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and led to significantly different changes of ALA, LA, EPA and ∑EPA + DHA concentrations in RBCs. In the course of the loLA/hiALA diet ALA and EPA concentrations and relative amounts of ∑EPA + DHA increased, whereas LA concentrations decreased. The DHA concentration was unaffected. The hiLA/loALA diet led to slightly decreased EPA concentrations, while all other fatty acid concentrations remained constant. Compared to our previous study, where we simply increased the ALA intake, our results show that ALA supplementation combined with a reduced LA intake (loLA/hiALA diet) more efficiently enhanced EPA blood concentrations. The absence of changes in the PUFA pattern in consequence of a LA/ALA ratio of 25.6 ± 2.41
1 and led to significantly different changes of ALA, LA, EPA and ∑EPA + DHA concentrations in RBCs. In the course of the loLA/hiALA diet ALA and EPA concentrations and relative amounts of ∑EPA + DHA increased, whereas LA concentrations decreased. The DHA concentration was unaffected. The hiLA/loALA diet led to slightly decreased EPA concentrations, while all other fatty acid concentrations remained constant. Compared to our previous study, where we simply increased the ALA intake, our results show that ALA supplementation combined with a reduced LA intake (loLA/hiALA diet) more efficiently enhanced EPA blood concentrations. The absence of changes in the PUFA pattern in consequence of a LA/ALA ratio of 25.6 ± 2.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 suggests that the high LA/ALA ratio of the Western diet already leads to a saturation and a further increase of the ratio does not affect the PUFA pattern.
1 suggests that the high LA/ALA ratio of the Western diet already leads to a saturation and a further increase of the ratio does not affect the PUFA pattern.
Introduction
The long chain (LC) omega-3 (n3) polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA, C20:5n3) and docosahexaenoic acid (DHA, C22:6n3) are known for their beneficial health effects mainly with regard to cardiovascular1–5 and cognitive health.6–8 Dietary sources of EPA and DHA are limited and in the Western diet intake of these fatty acids is far below the recommendations. Accordingly, blood levels of EPA and DHA are low.9The essential n3 precursor fatty acid alpha-linolenic acid (ALA, C18:3n3) is present in high amounts in some plant oils, particularly linseed-, chia-, perilla- and walnut oil and can be converted into EPA and DHA in a multistep elongation and desaturation reaction.10 However, the efficiency of this process is generally low in adult humans.11 It is, inter alia, influenced by the intake of the n6 precursor fatty acid linoleic acid (LA, C18:2n6) due to competition for the same desaturation and elongation enzymes12,13 and for incorporation into cell membranes.14 The intake of LA has increased substantially in Western diets during the last century.15,16 Consequently, the dietary ratio of the n6 fatty acid LA to the n3 fatty acid ALA is about 10–20:1 (ref. 17–19) which is viewed as unfavorable and may result in an inefficient conversion of ALA to the physiologically important n3 PUFAs EPA and DHA.12,13 A reduction of LA has been suggested to enhance the conversion of ALA to the longer chain n3 PUFAs EPA and DHA. Therefore, the biological efficacy of n3 PUFAs is improved and at the same time production of n6 derived pro-inflammatory mediators is decreased.20
The aim of our study was to compare two extreme ratios of LA to ALA involving a low-LA/high-ALA (loLA/hiALA) diet with a ratio of 0.5–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and a high-LA/low-ALA (hiLA/loALA) diet with a ratio of LA 20–30
1 and a high-LA/low-ALA (hiLA/loALA) diet with a ratio of LA 20–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on fatty acid concentrations in red blood cells (RBCs) with special emphasis on EPA and DHA. To achieve the two dietary LA/ALA ratios, the fatty acid supply of the subjects was tightly controlled via a multistep method. A homogenous collective of healthy men in a narrow age class was chosen to minimize potential fluctuations due to age21,22 and hormonal influences.23
1 on fatty acid concentrations in red blood cells (RBCs) with special emphasis on EPA and DHA. To achieve the two dietary LA/ALA ratios, the fatty acid supply of the subjects was tightly controlled via a multistep method. A homogenous collective of healthy men in a narrow age class was chosen to minimize potential fluctuations due to age21,22 and hormonal influences.23
Material and methods
Study design
This investigator-initiated study was conducted according to the guidelines laid down in the Declaration of Helsinki. The ethic committee at the medical chamber of Lower Saxony (Hannover, Germany) approved all procedures. Written informed consent was obtained from all subjects. The study is registered in the German clinical trial register (no. DRKS00011199).The study was conducted at the Institute of Food Science and Human Nutrition, Leibniz University Hannover, Germany. It consisted of a screening phase, two four-week run-in phases, a nine-week wash-out phase and two 14-day intervention phases (Fig. 1). In each intervention phase three examinations were carried out: at baseline (day 0), after 7 (day 7) and after 14 days (day 14). In the run-in phases the participants were requested to abstain from fish, seafood, and ALA-rich vegetable oils such as linseed oil or chia seeds. The aim of the intervention periods was to obtain two different dietary ratios of LA to ALA (0.5–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 20–30
1 and 20–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Due to the cross-over design, each subject acted as its own control, which minimizes interindividual variability of blood fatty acid levels as well as potential fluctuations regarding dietary intakes between individuals.
1). Due to the cross-over design, each subject acted as its own control, which minimizes interindividual variability of blood fatty acid levels as well as potential fluctuations regarding dietary intakes between individuals.
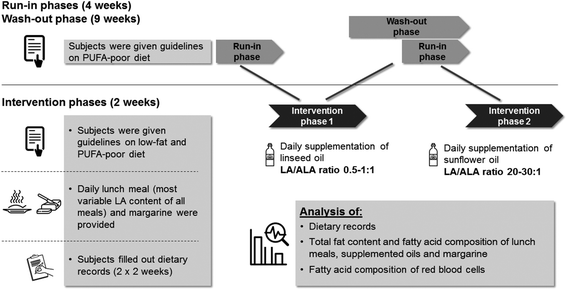 | ||
| Fig. 1 Schematic presentation of the study methods. ALA: α-linolenic acid; LA: linoleic acid; PUFA: polyunsaturated fatty acids. | ||
The control of the fatty acid intake was achieved by the following measures:
(A) Daily provision of the lunch meal by the Institute of Food Science and Human Nutrition, Leibniz University Hannover. The aim was to provide the same quantities of PUFAs to the participants. The daily freshly cooked lunch was low in LA and practically free of n3 fatty acids including ALA, EPA and DHA. The lunch meals were identical in both intervention phases. Subjects were allowed to eat lunch meals ad libitum. The consumed portions were all weighed.
(B) Complete replacement and standardization of the spreadable fat. Participants had to consume 60 g of provided margarine per week in both intervention phases, which should replace other spreadable fat. The margarine had a fat content of 74.3 g per 100 g with a LA content of 15.2% and an ALA content of 6.8%. Via margarine, participants therefore consumed 0.97 g LA and 0.43 g ALA per day.
(C) Daily supplementation of an ALA- and a LA-rich vegetable oil to adjust the intake of LA and ALA of the participants. In the intervention period with the loLA/hiALA diet subjects daily ingested 22.3 g of linseed oil with an ALA content of 55.9% of total fatty acids (Table 1) resulting in a daily ALA intake from linseed oil of 12.5 g per day. In the intervention period with the hiLA/loALA diet, subjects ingested a daily dose of 22.3 g of sunflower oil with a LA content of 62.5% (Table 1). Hence, daily LA intake from sunflower oil was 13.9 g per day.
| Fatty acid | Common name | Margarine | Linseed oil | Sunflower oil |
|---|---|---|---|---|
| C12:0 | Lauric acid | 6.2 | — | — |
| C14:0 | Myristic acid | 2.5 | — | — |
| C16:0 | Palmitic acid | 22.1 | 6.0 | 6.1 |
| C18:0 | Stearic acid | 2.4 | 4.9 | 3.2 |
| C18:1n9 | Oleic acid | 44.8 | 19.3 | 28.2 |
| C18:2n6 | Linoleic acid | 15.2 | 13.9 | 62.5 |
| C18:3n3 | α-Linolenic acid | 6.8 | 55.9 | — |
| C20:0 | Arachidic acid | — | — | — |
| C22:0 | Behenic acid | — | — | — |
| C24:0 | Lignoceric acid | — | — | — |
(D) Subjects were instructed to consume a low-fat and PUFA-poor diet containing no vegetable fats, but lots of fruit and vegetables, low-fat dairy products and white flour products. The subjects were provided with take-away foods (e.g. fruits, vegetable-soups, white bread) to help them comply with the PUFA-poor diet. The participants were requested to maintain their diet unchanged during both intervention phases.
The subjects had to record the consumed foods and drinks during both intervention phases in (daily) nutritional records. The nutritional records were checked daily for the requested nutrition, completeness, readability and plausibility by nutritionists and, if necessary, ambiguities were clarified directly with the subjects. The analysis of energy and nutrient intake was implemented using PRODI® (Nutri-Science GmbH, Freiburg, Germany). In addition, the fat content and the fatty acid composition of the provided lunch was analyzed by GC and included in the PRODI® analysis.
Study population
Participants were recruited from the general population in Hannover, Germany by advertisements. Subjects were pre-selected via screening questionnaires according to the following inclusion criteria: Male sex, age between 20 and 40 years, body mass index (BMI) between 20 and 27 kg m−2, mixed diet with low meat and fish consumption. Exclusion criteria were defined as followed: Smoking, serum triglyceride (TG) levels ≥150 mg dl−1 (≥1.7 mmol l−1); serum total cholesterol levels ≥200 mg dl−1 (≥5.2 mmol l−1); a relative amount of ∑EPA + DHA in red blood cells ≤3 and ≥6%, intake of fish (>2 times per week) as well as addiction to alcohol, drugs and/or medications and diseases: chronic diseases (e.g. malignant tumors, manifest cardiovascular disease, insulin-dependent type 1 and 2 diabetes, severe renal or liver diseases); chronic gastrointestinal disorders (especially small intestine, pancreas, liver) as well as prior gastrointestinal surgical procedures (e.g. gastrectomy); hormonal disorders (e.g. Cushing's syndrome and untreated hyperthyroidism); uncontrolled hypertension; blood coagulation disorders and intake of coagulation-inhibiting drugs; periodic intake of laxatives; intake of anti-inflammatory drugs (incl. acetylsalicylic acid); intake of lipid lowering drugs or supplements during the last 3 months before baseline examination. Inclusion and exclusion criteria were assessed via questionnaires. The pre-selected subjects were invited for a screening examination to collect fasting blood for the analysis of serum lipid levels, liver enzymes and fatty acid patterns in RBC.Proband examination, blood sampling and pre-analytical procedures
During each examination, fasting blood was collected, blood pressure was measured and subjects completed a questionnaire to obtain information about changes in medication and lifestyle habits (e.g. physical activity), as well as the tolerability of linseed and sunflower oil. Blood samples were obtained by venipuncture of an arm vein using Multiflyneedles (Sarstedt, Nümbrecht, Germany) into serum and EDTA monovettes (Sarstedt). All six examinations, including blood sampling, were performed at the same time for each subject. For analysis of fatty acids in RBCs, the cell sediment after centrifugation for 10 min at 1500g and 4 °C and removal of plasma was reconstituted in PBS to the initial blood volume, transferred into 1.5 mL Eppendorf tubes and immediately frozen and stored at −80 °C until extraction and analysis. All transfer steps were carried out on ice. Serum lipid levels, liver enzymes and small blood picture were determined in the LADR laboratory (Laborärztliche Arbeitsgemeinschaft für Diagnostik und Rationalisierung e.V.), Hannover, Germany.Fatty acid analyses
The total fat content of food samples was determined by gravimetry after lipid extraction according to Weibull-Stoldt performed as rapid microextraction.24 Fatty acids in blood cells were analyzed as fatty acid methyl esters (FAME) by means of gas chromatography with flame ionization detection (GC-FID) on a 6890 series GC instrument (Agilent, Waldbronn, Germany) as described25 with slight modifications. In brief, 10 μL internal standard (methyl pentacosanoate, FAME C25:0, 750 μM) was added to 100 μL of resuspended blood cells. Lipids were extracted with MTBE/MeOH and the lipid extract was derivatized with methanolic hydrogen chloride. The resulting FAMEs were separated on a FAMEWAX capillary column (30 m, 0.25 mm ID, 0.25 μm df; Restek, Bad Homburg, Germany) and quantification of FAMEs was based on response factors. Fatty acid concentrations in food samples were calculated as g fatty acid per 100 g fat. Fatty acid concentrations were quantified in whole blood cells, which are 99% RBCs. In RBC samples additionally to the concentration expressed as μg fatty acid per mL blood, the relative amount (% of total fatty acids) of each fatty acid was calculated directly based on peak areas.25Calculations and statistics
Results of anthropometrical measures, serum lipid levels and dietary energy and fat intake are stated as mean ± standard deviation (SD), while PUFA levels in RBCs and its relative change (%) are stated as mean ± standard error (SE). If the concentration of an analyte was below the lower limit of quantification (LLOQ) in more than 50% of the samples at one time point, the LLOQ is given for this analyte. Relative changes of the variables (v) were calculated individually for each subject at each time point (x) as Δ%, calculated by: Δ% = 100 × (vtx − vt0)/vt0.The distributions of the sample sets were analyzed by means of the Kolmogorov–Smirnov test. t-Tests for paired samples were used to determine statistical significance between the two interventions at baseline (day 0), after seven days (day 7) and after 14 days (day 14). To examine differences between the two interventions, two-factorial ANOVAs with repeated measurements of both factors were used. One-factorial ANOVAs with repeated measurements were carried out to examine the effect of time within the two interventions (day 0, day 7, day 14) separately for each intervention. Post-hoc t-tests for paired samples with Holm–Bonferroni-adjusted levels of significance were used to evaluate differences between the time points. Statistical significance was set at p ≤ 0.05 for all analyses. All statistical analyses were carried out with SPSS software (Version 24, SPSS Inc., Chicago, IL, USA).
Results
Study population
Fifteen male subjects met the criteria and thus were included in the study. All participants (mean age 26.1 ± 4.53 years) were healthy and had a normal BMI (24.0 ± 1.65 kg m−2) and serum lipid pattern (Table 3). Before the beginning of the first four-week run-in phase, the study collective consumed a normal mixed diet (including 2–3 portions of meat per week) with low fish consumption (≤1 serving fish per week) and low fruit and vegetable consumption (1–2 portions per day) and had a medium physical activity status (3–5 hours of sports per week) and a high education level (all participants had the general matriculation standard). All 15 participants completed the two intervention periods and attended at all six examinations.During the intervention periods, mean fruit and vegetable consumption increased to 5 portions per day, meat intake decreased to 2 portions per week and fish was not consumed during the intervention periods.
The examination of the dietary records combined with analysis of fatty acids in the lunch meal showed that the LA intake was 7.30 ± 0.37 g d−1 (2.78 en%) during loLA/hiALA diet and 18.2 ± 0.54 g d−1 (6.95 en%) during hiLA/loALA diet, while the ALA intake was 13.1 ± 0.22 g d−1 (4.98 en%) during loLA/hiALA diet and 0.71 ± 0.09 g d−1 (0.27 en%) during hiLA/loALA diet. The actual dietary LA/ALA ratios were therefore 0.56 ± 0.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the loLA/hiALA diet and 25.6 ± 2.41
1 in the loLA/hiALA diet and 25.6 ± 2.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the hiLA/loALA diet (Table 2). Intake of arachidonic acid (AA), EPA, DPAn3 and DHA was very low and did not differ between both intervention periods. Total PUFA intake was significantly higher during loLA/hiALA diet (22.5 ± 0.64 g d−1) compared to hiLA/loALA diet (18.5 ± 1.11 g d−1), while saturated fatty acid (SFA) and monounsaturated fatty acid (MUFA) intake were marginally, but significantly, higher during hiLA/loALA diet. However, fat intake as well as energy, protein, and carbohydrate intake did not differ between both intervention periods (Table 2).
1 in the hiLA/loALA diet (Table 2). Intake of arachidonic acid (AA), EPA, DPAn3 and DHA was very low and did not differ between both intervention periods. Total PUFA intake was significantly higher during loLA/hiALA diet (22.5 ± 0.64 g d−1) compared to hiLA/loALA diet (18.5 ± 1.11 g d−1), while saturated fatty acid (SFA) and monounsaturated fatty acid (MUFA) intake were marginally, but significantly, higher during hiLA/loALA diet. However, fat intake as well as energy, protein, and carbohydrate intake did not differ between both intervention periods (Table 2).
| loLA /hiALA | hiLA/loALA | t-Testa | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| AA: arachidonic acid; ALA: α-linolenic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; DPAn3: n3 docosapentaenoic acid; LA: linoleic acid; MUFA: monounsaturated fatty acids; n.s.: not significant; PUFA: polyunsaturated fatty acids; SFA: saturated fatty acids.a t-Test for paired samples; significance level p ≤ 0.05b Energy, protein, total fat, SFA, MUFA and PUFA intake were calculated from analyses of dietary records with PRODI®c LA, ALA, AA, EPA, DPAn3 and DHA intake were calculated from a combination of own analyses of meals that were provided by the Institute of Food Science and Human Nutrition and analyses of dietary records with PRODI®. | |||
| Energy intakeb (kcal) | 2444 ± 327 | 2436 ± 340 | n.s. |
| Proteinb (g) | 95.5 ± 16.1 | 95.9 ± 16.4 | n.s. |
| Carbohydratesb (g) | 325 ± 54.2 | 322 ± 54.2 | n.s. |
| Total fat intakeb (g) | 67.6 ± 5.43 | 68.5 ± 6.93 | n.s. |
| SFAb (g) | 17.3 ± 2.23 | 19.4 ± 2.26 | 0.040 |
| MUFAb (g) | 10.2 ± 0.93 | 11.8 ± 1.41 | <0.001 |
| PUFAb (g) | 22.5 ± 0.64 | 18.5 ± 1.11 | <0.001 |
| LAc (g) | 7.30 ± 0.37 | 18.2 ± 0.54 | <0.001 |
| ALAc (g) | 13.1 ± 0.22 | 0.71 ± 0.09 | <0.001 |
| LA/ALAc | 0.56 ± 0.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25.6 ± 2.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— |
| AAc (g) | 0.02 ± 0.01 | 0.02 ± 0.01 | n.s. |
| EPAc (g) | 0.00 ± 0.00 | 0.00 ± 0.00 | n.s. |
| DPAn3c (g) | 0.01 ± 0.01 | 0.01 ± 0.01 | n.s. |
| DHAc (g) | 0.03 ± 0.01 | 0.02 ± 0.01 | n.s. |
Clinical and anthropometric parameters of the participants were not significantly different between both interventions at baseline (Table 3). A slight decrease in body weight, BMI, total cholesterol (TC), low density lipoprotein (LDL) and high density lipoprotein (HDL) was observed in both intervention periods. During hiLA/loALA diet, but not during loLA/hiALA diet, the diastolic blood pressure decreased. All other parameters remained constant in both intervention periods (Table 3).
| loLA/hiALA | An reM | hiLA/loALA | An reM | |||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 0 | Day 7 | Day 14 | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Levels are shown at day 0, 7 and 14 of loLA/hiALA diet and hiLA/loALA diet. An reM: ANOVA for repeated measures; BMI: body mass index; dias BP: diastolic blood pressure; HDL: high density lipoprotein; LDL: low density lipoprotein; SD: standard deviation; sys BP: systolic blood pressure; TC: total cholesterol; TG: triglycerides. aSignificant difference between loLA/hiALA diet week 0 and hiLA/loALA diet week 0 (t-test for paired samples). bSignificant difference between loLA/hiALA diet week 1 and hiLA/loALA diet week 1 (t-test for paired samples). cSignificant difference between loLA/hiALA diet week 2 and hiLA/loALA diet week 2 (t-test for paired samples). dSignificant difference between week 0 and week 2 of loLA/hiALA diet /hiLA/loALA diet (t-test for paired samples). eSignificant difference within loLA/hiALA diet (one-factorial ANOVA for repeated measures). fSignificant difference within hiLA/loALA diet (one-factorial ANOVA for repeated measures). gSignificant difference within loLA/hiALA diet between week 0 and week 1 (t-test for paired samples with Holm–Bonferroni correction). hSignificant difference within loLA/hiALA diet between week 0 and week 2 (t-test for paired samples with Holm–Bonferroni correction). iSignificant difference within hiLA/loALA between week 0 and week 1 (t-test for paired samples with Holm–Bonferroni correction). kSignificant difference within hiLA/loALA between week 0 and week 2 (t-test for paired samples with Holm–Bonferroni correction). lSignificant difference between loLA/hiALA diet und hiLA/loALA (two-factorial ANOVA for repeated measures). *p ≤ 0.05, #p ≤ 0.005, ‡p < 0.001. | ||||||||
| Age (years) | 26.1 ± 4.53 | |||||||
| Weight (kg) | 81.4 ± 7.44 | 80.1 ± 7.49d‡ | 81.9 ± 8.11 | 80.9 ± 8.09d‡ | ||||
| BMI (kg m−2) | 24.0 ± 1.65 | 23.6 ± 1.70d‡ | 24.2 ± 1.81 | 23.9 ± 1.82d‡ | ||||
| Sys BP (mmHg) | 127 ± 7.99 | 124 ± 10.7 | 125 ± 12.0 | 129 ± 11.0 | 124 ± 11.8 | 126 ± 13.2 | ||
| Dias BP (mmHg) | 77.7 ± 4.58 | 77.7 ± 5.30b* | 76.0 ± 6.32 | 78.0 ± 6.21 | 73.7 ± 6.11b* | 74.0 ± 5.73k* | f* | |
| TC (mg dl−1) | 177 ± 42.8 | 155 ± 32.4g# | 150 ± 27.0h# | e‡ | 169 ± 34.8 | 148 ± 30.7i‡ | 152 ± 37.9k* | f# |
| HDL (mg dl−1) | 56.5 ± 9.64 | 51.5 ± 9.53g* | 49.7 ± 8.78h* | e‡ | 58.9 ± 9.77 | 51.5 ± 7.83i‡ | 53.0 ± 9.82 | f# |
| LDL (mg dl−1) | 110 ± 31.4 | 97.9 ± 26.4b*, g* | 93.7 ± 18.9h* | e# | 106 ± 28.5 | 91.0 ± 24.5b*, i‡ | 91.8 ± 27.5k# | f‡ |
| TG (mg dl−1) | 106 ± 50.8 | 100 ± 44.6 | 107 ± 46.7 | 108 ± 36.0 | 98.5 ± 31.3 | 99.9 ± 54.8 | ||
Changes of fatty acid patterns in RBCs
At baseline, there were only a few marginal differences in the fatty acid patterns of RBCs between both intervention phases (Table S1†). Prior interventions, AA was present in highest concentrations in RBCs (loLA/hiALA diet: 152 ± 4.08 μg mL−1; hiLA/loALA diet: 145 ± 4.10 μg mL−1) among all PUFAs, followed by LA (loLA/hiALA diet: 101 ± 3.78 μg mL−1; hiLA/loALA diet: 99.1 ± 2.89 μg mL−1), DHA (loLA/hiALA diet: 36.1 ± 1.75 μg mL−1; hiLA/loALA diet: 34.3 ± 2.04 μg mL−1), C22:4n6 (loLA/hiALA diet: 32.5 ± 1.58 μg mL−1; hiLA/loALA diet: 29.5 ± 0.92 μg mL−1), DPAn3 (loLA/hiALA diet: 27.3 ± 1.42 μg mL−1; hiLA/loALA diet: 28.0 ± 0.98 μg mL−1), DPAn6 (loLA/hiALA diet: 5.84 ± 0.35 μg mL−1; hiLA/loALA diet: 5.12 ± 0.24 μg mL−1) and EPA (loLA/hiALA diet: 5.49 ± 0.48 μg mL−1; hiLA/loALA diet: 5.86 ± 0.41 μg mL−1). ALA concentrations in RBCs were low at baseline of loLA/hiALA diet and hiLA/loALA diet with 1.44 ± 0.17 μg mL−1 and 1.47 ± 0.13 μg mL−1, respectively (Table 4 & 5).| Day 0 | Day 7 | t-Testa | Day 14 | t-Testa | 1-fact. An reMb | |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | p (day 7–day 0) | Mean ± SE | p (day 14–day 0) | p | |
| Levels are shown as concentration [μg mL−1] in blood and as relative amount [%] of total fatty acids. An reM: ANOVA for repeated measures; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; MUFA: monounsaturated fatty acids: C14:1n5, C15:1n5, C16:1n7, C17:1n7, C18:1n9, C18:1n7, C20:1n9, C22:1n9, 24:1n9; n.s.: not significant; SFA: saturated fatty acids: C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C21:0, C22:0, C24:0; PUFA: polyunsaturated fatty acids: C18:2n6, C18:3n6, C18:3n3, C:18:4n3, C20:2n6, C20:3n3, C20:3n6, C20:3n9, C20:4n3, C20:4n6, C20:5n3, C22:2n6, C22:4n6, C22:5n3, C22:5n6, C22:6n3; TFA: total fatty acids.a t-Test for paired samples with Holm–Bonferroni correction (within intervention); significance level p ≤ 0.05b One-factorial ANOVA for repeated measures (An reM); significance level p ≤ 0.05. | ||||||
| C10:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C11:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C12:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C13:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C14:0 (μg mL−1) | 3.31 ± 0.16 | 3.15 ± 0.17 | — | 2.78 ± 0.21 | — | n.s. |
| % of total FA | 0.32 ± 0.02 | 0.31 ± 0.01 | — | 0.30 ± 0.02 | — | n.s. |
| C14:1n5 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C15:0 (μg mL−1) | 1.59 ± 0.08 | 1.61 ± 0.06 | — | 1.37 ± 0.07 | — | n.s. |
| % of total FA | 0.15 ± 0.01 | 0.16 ± 0.00 | — | 0.15 ± 0.01 | — | n.s. |
| C15:1n5 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C16:0 (μg mL−1) | 212 ± 7.22 | 209 ± 5.89 | n.s. | 184 ± 6.24 | n.s. | 0.017 |
| % of total FA | 20.4 ± 0.18 | 20.7 ± 0.13 | n.s. | 19.8 ± 0.18 | n.s. | 0.005 |
| C16:1n7 (μg mL−1) | 2.88 ± 0.18 | 3.11 ± 0.32 | — | 2.89 ± 0.37 | — | n.s. |
| % of total FA | 0.28 ± 0.02 | 0.30 ± 0.02 | — | 0.31 ± 0.03 | — | n.s. |
| C17:0 (μg mL−1) | 3.10 ± 0.12 | 3.07 ± 0.08 | <0.001 | 2.76 ± 0.08 | n.s. | <0.001 |
| % of total FA | 0.30 ± 0.01 | 0.31 ± 0.01 | <0.001 | 0.30 ± 0.01 | n.s. | <0.001 |
| C17:1n8 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C18:0 (μg mL−1) | 157 ± 4.53 | 154 ± 3.82 | — | 141 ± 3.85 | — | n.s. |
| % of total FA | 15.2 ± 0.18 | 15.3 ± 0.13 | — | 15.2 ± 0.15 | — | n.s. |
| C18:1n9 (μg mL−1) | 139 ± 4.78 | 130 ± 4.69 | n.s. | 117 ± 5.25 | n.s. | 0.016 |
| % of total FA | 13.4 ± 0.18 | 12.8 ± 0.21 | 0.001 | 12.6 ± 0.25 | <0.001 | <0.001 |
| C18:1n7 (μg mL−1) | 14.5 ± 0.59 | 14.3 ± 0.42 | — | 13.5 ± 0.33 | — | n.s. |
| % of total FA | 1.40 ± 0.02 | 1.42 ± 0.02 | n.s. | 1.45 ± 0.02 | n.s. | 0.019 |
| C18:2n6 (μg mL−1) | 101 ± 3.78 | 91.6 ± 2.59 | n.s. | 82.8 ± 2.75 | n.s. | 0.029 |
| % of total FA | 9.72 ± 0.20 | 9.12 ± 0.19 | n.s. | 8.90 ± 0.15 | n.s. | 0.012 |
| C18:3n6 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C19:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C18:3n3 (μg mL−1) | 1.44 ± 0.17 | 5.63 ± 0.45 | <0.001 | 6.34 ± 0.63 | <0.001 | <0.001 |
| % of total FA | 0.14 ± 0.02 | 0.55 ± 0.03 | <0.001 | 0.67 ± 0.05 | <0.001 | <0.001 |
| C18:4n3 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:0 (μg mL−1) | 3.87 ± 0.14 | 3.92 ± 0.12 | — | 3.71 ± 0.14 | — | n.s. |
| % of total FA | 0.37 ± 0.01 | 0.39 ± 0.01 | — | 0.40 ± 0.01 | — | n.s. |
| C20:1n9 (μg mL−1) | 2.86 ± 0.21 | 2.79 ± 0.13 | — | 2.52 ± 0.10 | — | n.s. |
| % of total FA | 0.27 ± 0.01 | 0.28 ± 0.01 | — | 0.27 ± 0.01 | — | n.s. |
| C20:2n6 (μg mL−1) | 2.00 ± 0.12 | 1.87 ± 0.10 | — | 1.62 ± 0.07 | — | n.s. |
| % of total FA | 0.19 ± 0.01 | 0.19 ± 0.01 | — | 0.18 ± 0.01 | — | n.s. |
| C20:3n9 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:3n6 (μg mL−1) | 15.9 ± 0.93 | 14.1 ± 0.96 | — | 12.9 ± 0.94 | — | n.s. |
| % of total FA | 1.53 ± 0.07 | 1.39 ± 0.08 | — | 1.38 ± 0.10 | — | n.s. |
| C21:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:4n6 (μg mL−1) | 152 ± 4.08 | 147 ± 3.61 | — | 139 ± 3.16 | — | n.s. |
| % of total FA | 14.7 ± 0.18 | 14.7 ± 0.20 | — | 15.0 ± 0.27 | — | n.s. |
| C20:3n3 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:4n3 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:5n3 (μg mL−1) | 5.49 ± 0.48 | 6.97 ± 0.55 | 0.019 | 8.27 ± 0.82 | 0.008 | 0.002 |
| % of total FA | 0.53 ± 0.04 | 0.69 ± 0.05 | 0.009 | 0.88 ± 0.08 | <0.001 | <0.001 |
| C22:0 (μg mL−1) | 16.6 ± 0.36 | 16.5 ± 0.54 | — | 15.5 ± 0.49 | — | n.s. |
| % of total FA | 1.61 ± 0.04 | 1.64 ± 0.03 | — | 1.66 ± 0.04 | — | n.s. |
| C22:1n9 (μg mL−1) | 1.56 ± 0.20 | 2.08 ± 0.28 | — | 1.94 ± 0.34 | — | n.s. |
| % of total FA | 0.15 ± 0.02 | 0.20 ± 0.03 | — | 0.21 ± 0.04 | — | n.s. |
| C22:2n6 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C22:4n6 (μg mL−1) | 32.5 ± 1.58 | 30.7 ± 1.21 | — | 27.9 ± 1.11 | — | n.s. |
| % of total FA | 3.13 ± 0.11 | 3.06 ± 0.11 | — | 3.01 ± 0.11 | — | n.s. |
| C22:5n6 (μg mL−1) | 5.84 ± 0.35 | 5.49 ± 0.34 | — | 5.11 ± 0.32 | — | n.s. |
| % of total FA | 0.56 ± 0.03 | 0.54 ± 0.03 | — | 0.55 ± 0.03 | — | n.s. |
| C22:5n3 (μg mL−1) | 27.3 ± 1.42 | 27.1 ± 1.05 | — | 27.1 ± 1.34 | — | n.s. |
| % of total FA | 2.62 ± 0.09 | 2.70 ± 0.09 | n.s. | 2.91 ± 0.10 | 0.004 | 0.002 |
| C24:0 (μg mL−1) | 47.1 ± 1.14 | 45.9 ± 1.21 | — | 44.1 ± 1.29 | — | n.s. |
| % of total FA | 4.56 ± 0.06 | 4.56 ± 0.05 | n.s. | 4.75 ± 0.06 | n.s. | 0.031 |
| C22:6n3 (μg mL−1) | 36.1 ± 1.75 | 35.8 ± 1.75 | — | 35.8 ± 1.44 | — | n.s. |
| % of total FA | 3.50 ± 0.15 | 3.56 ± 0.16 | — | 3.87 ± 0.16 | — | n.s. |
| C24:1n9 (μg mL−1) | 51.9 ± 2.42 | 50.7 ± 1.73 | — | 49.1 ± 1.34 | — | n.s. |
| % of total FA | 4.99 ± 0.13 | 5.04 ± 0.11 | — | 5.30 ± 0.14 | — | n.s. |
| ∑TFA (μg mL−1) | 1040 ± 30.9 | 1010 ± 25.9 | — | 933 ± 25.8 | — | n.s. |
| ∑SFA (μg mL−1) | 446 ± 13.1 | 438 ± 11.1 | n.s. | 396 ± 11.5 | n.s. | 0.031 |
| % of total FA | 42.9 ± 0.30 | 43.4 ± 0.13 | n.s. | 42.5 ± 0.18 | n.s. | 0.025 |
| ∑MUFA (μg mL−1) | 213 ± 7.87 | 203 ± 6.87 | n.s. | 188 ± 6.85 | n.s. | 0.039 |
| % of total FA | 20.5 ± 0.31 | 20.1 ± 0.27 | n.s. | 20.1 ± 0.32 | n.s. | 0.036 |
| ∑PUFA (μg mL−1) | 381 ± 11.0 | 369 ± 8.72 | — | 349 ± 8.62 | — | n.s. |
| % of total FA | 36.7 ± 0.19 | 36.5 ± 0.20 | n.s. | 37.5 ± 0.28 | n.s. | 0.002 |
| ∑EPA + DHA (μg mL−1) | 41.6 ± 2.04 | 42.8 ± 2.04 | — | 44.1 ± 1.96 | — | n.s. |
| % of total FA | 4.03 ± 0.17 | 4.25 ± 0.18 | n.s. | 4.76 ± 0.20 | 0.019 | 0.001 |
| Day 0 | Day 7 | t-Testa | Day 14 | t-Testa | 1-fact. An reMb | |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | p (day 7–day 0) | Mean ± SE | p (day 14–day 0) | p | |
| Levels are shown as concentration [μg mL−1] in blood and as relative amount [%] of total fatty acids. An reM: ANOVA for repeated measures; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; MUFA: monounsaturated fatty acids: C14:1n5, C15:1n5, C16:1n7, C17:1n7, C18:1n9, C18:1n7, C20:1n9, C22:1n9, 24:1n9; n.s.: not significant; SFA: saturated fatty acids: C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C21:0, C22:0, C24:0; PUFA: polyunsaturated fatty acids: C18:2n6, C18:3n6, C18:3n3, C:18:4n3, C20:2n6, C20:3n3, C20:3n6, C20:3n9, C20:4n3, C20:4n6, C20:5n3, C22:2n6, C22:4n6, C22:5n3, C22:5n6, C22:6n3; TFA: total fatty acids.a t-Test for paired samples with Holm–Bonferroni correction (within intervention); significance level p ≤ 0.05b One-factorial ANOVA for repeated measures (An reM); significance level p ≤ 0.05. | ||||||
| C10:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C11:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C12:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C13:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C14:0 (μg mL−1) | 3.07 ± 0.17 | 3.06 ± 0.12 | n.s. | 3.53 ± 0.21 | 0.029 | 0.006 |
| % of total FA | 0.31 ± 0.01 | 0.32 ± 0.01 | — | 0.34 ± 0.01 | — | n.s. |
| C14:1n5 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C15:0 (μg mL−1) | 1.51 ± 0.05 | 1.51 ± 0.05 | n.s. | 1.70 ± 0.07 | 0.045 | 0.008 |
| % of total FA | 0.15 ± 0.00 | 0.16 ± 0.00 | n.s. | 0.16 ± 0.01 | n.s. | 0.041 |
| C15:1n5 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C16:0 (μg mL−1) | 200 ± 4.48 | 194 ± 4.23 | n.s. | 218 ± 6.47 | 0.043 | 0.001 |
| % of total FA | 20.1 ± 0.13 | 20.5 ± 0.11 | <0.001 | 21.0 ± 0.13 | <0.001 | <0.001 |
| C16:1n7 (μg mL−1) | 2.87 ± 0.13 | 2.98 ± 0.16 | n.s. | 3.67 ± 0.47 | n.s. | 0.041 |
| % of total FA | 0.29 ± 0.01 | 0.32 ± 0.02 | — | 0.35 ± 0.04 | — | n.s. |
| C17:0 (μg mL−1) | 2.96 ± 0.09 | 2.87 ± 0.06 | <0.001 | 3.10 ± 0.08 | n.s. | <0.001 |
| % of total FA | 0.30 ± 0.01 | 0.30 ± 0.01 | <0.001 | 0.30 ± 0.01 | n.s. | <0.001 |
| C17:1n8 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C18:0 (μg mL−1) | 151 ± 3.50 | 142 ± 3.20 | 0.018 | 154 ± 4.25 | n.s. | 0.017 |
| % of total FA | 15.3 ± 0.10 | 15.1 ± 0.12 | n.s. | 14.8 ± 0.15 | 0.029 | 0.009 |
| C18:1n9 (μg mL−1) | 130 ± 2.65 | 121 ± 2.56 | 0.005 | 133 ± 5.29 | n.s. | 0.011 |
| % of total FA | 13.1 ± 0.20 | 12.8 ± 0.18 | 0.011 | 12.7 ± 0.25 | n.s. | 0.036 |
| C18:1n7 (μg mL−1) | 13.8 ± 0.34 | 13.5 ± 0.30 | n.s. | 15.2 ± 0.53 | n.s. | 0.008 |
| % of total FA | 1.39 ± 0.02 | 1.44 ± 0.02 | n.s. | 1.46 ± 0.03 | n.s. | 0.022 |
| C18:2n6 (μg mL−1) | 99.1 ± 2.89 | 94.5 ± 2.92 | n.s. | 110 ± 3.51 | n.s. | 0.001 |
| % of total FA | 9.99 ± 0.24 | 10.0 ± 0.23 | n.s. | 10.6 ± 0.22 | n.s. | 0.011 |
| C18:3n6 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C19:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C18:3n3 (μg mL−1) | 1.47 ± 0.13 | 1.09 ± 0.09 | 0.011 | 1.41 ± 0.17 | n.s. | 0.008 |
| % of total FA | 0.15 ± 0.01 | 0.12 ± 0.01 | 0.023 | 0.13 ± 0.01 | n.s. | 0.016 |
| C18:4n3 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:0 (μg mL−1) | 4.06 ± 0.11 | 3.70 ± 0.13 | — | 3.95 ± 0.15 | — | n.s. |
| % of total FA | 0.41 ± 0.01 | 0.39 ± 0.01 | — | 0.38 ± 0.01 | — | n.s. |
| C20:1n9 (μg mL−1) | 2.73 ± 0.13 | 2.57 ± 0.13 | 0.038 | 2.87 ± 0.12 | n.s. | 0.010 |
| % of total FA | 0.28 ± 0.01 | 0.27 ± 0.01 | — | 0.28 ± 0.01 | — | n.s. |
| C20:2n6 (μg mL−1) | 1.89 ± 0.08 | 1.85 ± 0.09 | — | 2.21 ± 0.11 | — | n.s. |
| % of total FA | 0.19 ± 0.01 | 0.20 ± 0.01 | — | 0.21 ± 0.01 | — | n.s. |
| C20:3n9 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:3n6 (μg mL−1) | 14.5 ± 0.82 | 14.1 ± 0.82 | n.s. | 15.9 ± 0.89 | n.s. | 0.010 |
| % of total FA | 1.47 ± 0.08 | 1.50 ± — | n.s. | 1.54 ± 0.09 | — | n.s. |
| C21:0 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:4n6 (μg mL−1) | 145 ± 4.10 | 138 ± 3.33 | n.s. | 151 ± 3.86 | n.s. | 0.011 |
| % of total FA | 14.6 ± 0.19 | 14.7 ± 0.14 | — | 14.6 ± 0.20 | — | n.s. |
| C20:3n3 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:4n3 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C20:5n3 (μg mL−1) | 5.86 ± 0.41 | 5.21 ± 0.35 | 0.002 | 5.11 ± 0.41 | 0.025 | 0.001 |
| % of total FA | 0.59 ± 0.04 | 0.55 ± 0.04 | 0.001 | 0.49 ± 0.03 | <0.001 | <0.001 |
| C22:0 (μg mL−1) | 16.5 ± 0.55 | 15.28 ± 0.54 | 0.025 | 16.6 ± 0.51 | n.s. | 0.017 |
| % of total FA | 1.66 ± 0.04 | 1.62 ± 0.03 | — | 1.60 ± 0.04 | — | n.s. |
| C22:1n9 (μg mL−1) | 2.03 ± 0.20 | 1.83 ± 0.21 | n.s. | 1.30 ± 0.12 | n.s. | 0.033 |
| % of total FA | 0.21 ± 0.02 | 0.19 ± 0.02 | n.s. | 0.13 ± 0.01 | n.s. | 0.023 |
| C22:2n6 (μg mL−1) | <0.25 | <0.25 | — | <0.25 | — | — |
| % of total FA | — | — | — | — | — | — |
| C22:4n6 (μg mL−1) | 29.5 ± 0.92 | 28.58 ± 1.25 | n.s. | 30.4 ± 1.13 | n.s. | 0.014 |
| % of total FA | 2.98 ± 0.11 | 3.03 ± 0.11 | — | 2.93 ± 0.09 | — | n.s. |
| C22:5n6 (μg mL−1) | 5.12 ± 0.24 | 5.04 ± 0.29 | n.s. | 5.42 ± 0.25 | n.s. | 0.015 |
| % of total FA | 0.52 ± 0.02 | 0.53 ± 0.03 | — | 0.52 ± 0.02 | — | n.s. |
| C22:5n3 (μg mL−1) | 28.0 ± 0.98 | 27.03 ± 0.91 | — | 27.9 ± 1.51 | — | n.s. |
| % of total FA | 2.82 ± 0.09 | 2.87 ± 0.08 | n.s. | 2.67 ± 0.10 | 0.021 | 0.001 |
| C24:0 (μg mL−1) | 46.7 ± 1.11 | 44.00 ± 1.36 | 0.032 | 46.6 ± 1.48 | n.s. | n.s. |
| % of total FA | 4.70 ± 0.06 | 4.65 ± 0.07 | n.s. | 4.48 ± 0.08 | 0.020 | 0.001 |
| C22:6n3 (μg mL−1) | 34.3 ± 2.04 | 32.4 ± 1.75 | n.s. | 35.68 ± 1.74 | n.s. | 0.024 |
| % of total FA | 3.44 ± 0.16 | 3.42 ± 0.15 | — | 3.44 ± 0.16 | — | n.s. |
| C24:1n9 (μg mL−1) | 49.9 ± 1.45 | 47.7 ± 1.66 | n.s. | 49.9 ± 1.64 | n.s. | n.s. |
| % of total FA | 5.03 ± 0.11 | 5.04 ± 0.11 | n.s. | 4.81 ± 0.12 | 0.031 | 0.001 |
| ∑TFA (μg mL−1) | 996 ± 20.6 | 947 ± 20.6 | 0.027 | 1043 ± 28.2 | n.s. | 0.003 |
| ∑SFA (μg mL−1) | 428 ± 9.33 | 408 ± 9.05 | 0.045 | 449 ± 12.3 | n.s. | 0.008 |
| % of total FA | 42.9 ± 0.15 | 43.0 ± 0.14 | — | 43.1 ± 0.19 | — | n.s. |
| ∑MUFA (μg mL−1) | 202 ± 4.06 | 190 ± 4.23 | 0.009 | 206 ± 7.00 | n.s. | 0.013 |
| % of total FA | 20.3 ± 0.25 | 20.1 ± 0.21 | 0.028 | 19.7 ± 0.26 | 0.012 | 0.003 |
| ∑PUFA (μg mL−1) | 367 ± 8.40 | 350 ± 7.94 | 0.039 | 387 ± 9.65 | n.s. | 0.002 |
| % of total FA | 36.8 ± 0.23 | 36.9 ± 0.16 | n.s. | 37.2 ± 0.19 | 0.015 | 0.021 |
| ∑EPA + DHA (μg mL−1) | 40.2 ± 2.27 | 37.6 ± 1.95 | — | 40.8 ± 1.91 | — | n.s. |
| % of total FA | 4.03 ± 0.18 | 3.97 ± 0.17 | n.s. | 3.93 ± 0.17 | 0.031 | 0.005 |
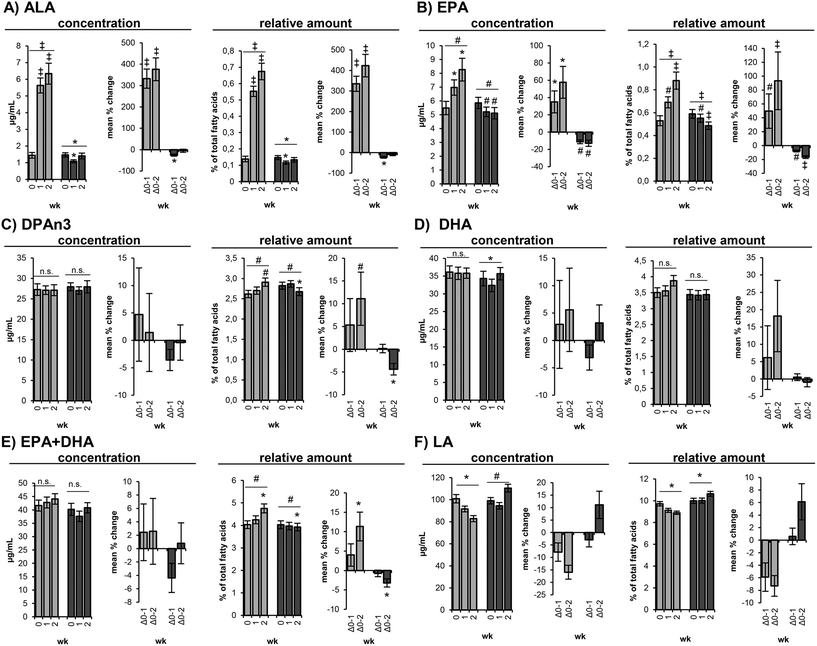
The two different dietary ratios of LA to ALA led to significantly different changes of the PUFA concentrations of ALA, LA, EPA and ∑EPA + DHA (Table S1†). In the following, the fatty acid concentrations are discussed unless the relative fatty acid distribution showed a different trend.
ALA
In the course of the loLA/hiALA diet ALA concentrations increased rapidly (p < 0.001) from 1.44 ± 0.17 μg mL−1 at baseline to 5.63 ± 0.45 μg mL−1 at day 7 and to 6.34 ± 0.63 μg mL−1 at day 14, corresponding to a mean change of 332 ± 40% and 354 ± 47% (Table 4 and Fig. 2A), whereas during hiLA/loALA diet ALA concentrations dropped after 7 days from 1.47 ± 0.13 μg mL−1 to 1.09 ± 0.09 μg mL−1 (p = 0.011) and increased again at day 14 to 1.41 ± 0.17 μg mL−1, which is not significantly different from the baseline level (Table 5 and Fig. 2A).LA
In the course of the loLA/hiALA diet a linear non-significant decrease of LA concentrations was observed between baseline (101 ± 3.78 μg mL−1), day 7 (91.6 ± 2.59 μg mL−1) and day 14 (82.8 ± 2.75 μg mL−1) (Table 4 and Fig. 2F). Following the hiLA/loALA diet, LA concentrations non-significantly increased from 99.1 ± 2.89 μg mL−1 at baseline to 110 ± 3.51 μg mL−1 at day 14 (Table 5 and Fig. 2F).EPA
EPA concentrations increased during loLA/hiALA diet from 5.49 ± 0.48 μg mL−1 at baseline to 6.97 ± 0.55 μg mL−1 at day 7 (p = 0.019) and to 8.27 ± 0.82 μg mL−1 at day 14 (p = 0.008), corresponding to a mean change of 35.0 ± 13% and 57.6 ± 18% (Table 4 and Fig. 2B). During hiLA/loALA diet EPA concentrations decreased from 5.86 ± 0.41 μg mL−1 at baseline to 5.21 ± 0.35 μg mL−1 at day 7 (p = 0.002) and 5.11 ± 0.41 μg mL−1 at day 14 (p = 0.025), corresponding to a mean change of −11.2 ± 2.1% and −12.9 ± 3.6% (Table 5 and Fig. 2B). Differences in EPA concentrations between both interventions at time point day 7 and day 14 were highly significant (p < 0.001) (Table S1†).DPAn3 and DPAn6
DPAn3 concentrations remained constant during both intervention phases (Tables 4, 5 & S1,†Fig. 2C). However, the relative DPAn3 amount slightly increased (p = 0.004) during loLA/hiALA diet from 2.62 ± 0.09% of total fatty acids at baseline to 2.91 ± 0.10% at day 14 (Table 4 and Fig. 2C) and slightly decreased (p = 0.021) during hiLA/loALA diet from 2.82 ± 0.09% at baseline to 2.67 ± 0.10% at day 14 (Table 5 and Fig. 2C). However, it has to be noted that baseline relative amounts of DPAn3 were significantly different (p = 0.005) between loLA/hiALA and hiLA/loALA diet (Table S1†).DPAn6 concentrations remained unchanged during loLA/hiALA and hiLA/loALA diet (Tables 4, 5 & S1†). Again, significantly (p = 0.026) different baseline DPAn6 concentrations were observed between loLA/hiALA and hiLA/loALA diet (Table S1†).
DHA
Also DHA concentrations remained unchanged in both intervention phases (Tables 4, 5 & S1,†Fig. 2D), even though the two-factorial ANOVA detected a significant difference (p = 0.025) between the loLA/hiALA and the hiLA/loALA diet (Table S1†) and DHA concentrations were significantly different (p = 0.006) at day 7 of loLA/hiALA diet (35.8 ± 1.75 μg mL−1) and hiLA/loALA diet (32.4 ± 1.75 μg mL−1) (Table S1†).AA
AA concentrations did not change significantly in both intervention periods (Tables 4, 5 & S1†). However, in the course of the loLA/hiALA diet AA concentrations slightly decreased (n.s.) from 152 ± 4.08 μg mL−1 (baseline) to 147 ± 3.61 μg mL−1 (day 7) and to 139 ± 3.16 μg mL−1 (day 14) (Table 4), whereas during the hiLA/loALA diet AA concentrations increased (n.s.) marginally from 145 ± 4.10 μg mL−1 (baseline) to 151 ± 3.86 μg mL−1 (day 14) (Table 5). These opposite trends are supported by significantly lower (p = 0.007) AA concentrations at day 14 of loLA/hiALA diet (139 ± 3.16 μg mL−1) compared to day 14 of hiLA/loALA diet (151 ± 3.86 μg mL−1) (Table S1†).∑EPA + DHA
The concentration of ∑EPA + DHA in RBCs increased slightly (n.s.) in response to the 14-day loLA/hiALA diet from 41.6 ± 2.04 μg mL−1 (baseline) to 42.8 ± 2.04 μg mL−1 (day 7) and to 44.1 ± 1.96 μg mL−1 (day 14) (Table 4 and Fig. 2E). The relative amount of ∑EPA + DHA in RBCs increased marginally but significantly (p = 0.019) in response to the loLA/hiALA diet from initially 4.03 ± 0.17% of total fatty acids to 4.76 ± 0.20% of total fatty acids at day 14 (Table 4 and Fig. 2E). In the course of the hiLA/loALA diet the concentration of ∑EPA + DHA in RBCs remained constant, though, the relative amount of ∑EPA + DHA in RBCs decreased slightly (p = 0.031) from 4.03 ± 0.18% of total fatty acids to 3.93 ± 0.17% at day 14 (Table 5 and Fig. 2E).Discussion
Study design
The design of clinical trials to investigate the effect of different dietary LA/ALA ratios on PUFA concentrations in blood is challenging. Although ALA is only present in a few plant oils and its intake can easily be controlled, LA is nowadays ubiquitous in our daily diet due to the widespread use of LA-rich vegetable oils especially in ready meals and take-away foods. This makes attempts to reduce the LA intake of free-living individuals difficult.26Numerous human studies have attempted to establish a defined dietary ratio of the precursor fatty acids LA and ALA to investigate the effect on the n3 PUFA status.20,27–44
According to a current review from Wood et al.,26 the majority of these studies, exhibited some or several methodological weaknesses such as studying males and females together,28–36 inappropriate or missing run-in and wash-out periods,28,39,43 inconsistent composition of the background diet, e.g. changes of EPA and DHA intake during interventions,31,37 inaccurate dietary records.29,31,33–35,37 These methodological weaknesses question the control and documentation of the PUFA intake and limit the ability to draw robust conclusions.26 Besides controlled PUFA intake and study conditions, several other factors have been proposed to influence ALA conversion in humans including age,21,22 BMI,22 smoking status,45 sex46–49 and genotype.13,50 For example, males and females are known to have different capacities for ALA conversion.13 Therefore, it is suggested that males and females are either stratified or studied separately.26
The aim of our study was to compare the effects of two different dietary LA/ALA ratios on fatty acid concentrations in RBC with special emphasis on LC n3 PUFAs. A homogenous study collective of healthy, non-smoking men within a narrow range regarding age (mean age 26.1 ± 4.53 years) and BMI (24.0 ± 1.65 kg m−2) was chosen to prevent/minimize the influence of gender, smoking, age, and BMI on PUFA metabolism. Likewise, our study approach includes further methodological considerations with the aim to overcome methodological weaknesses listed above. This involves in particular constant background diet and defined low-variable PUFA intake as well as subjects acting as their own controls (cross-over design), and run-in and wash-out phase. Other studies with the aim to modify LA and ALA intake mostly supplemented margarine and/or plant oils additionally to the normal background nutrition of the participants to achieve the desired ratio of LA to ALA.20,28,29,32,33,38–41,43 Studies, where subjects acted as their own controls are rare.20,39
With the two experimental diets a low-LA (2.78 en%) and a high-ALA (4.98 en%) diet and a high-LA (6.95 en%) and low-ALA (0.27 en%) diet were achieved, which correspond to a LA/ALA ratio of 0.56 ± 0.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 25.6 ± 2.41
1 and 25.6 ± 2.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. These ratios can be classified as extreme examples of a desirable presumably health-promotive LA/ALA ratio (loLA/hiALA diet, <5
1, respectively. These ratios can be classified as extreme examples of a desirable presumably health-promotive LA/ALA ratio (loLA/hiALA diet, <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and an unfavorable LA/ALA ratio (hiLA/loALA diet, ≫5
1) and an unfavorable LA/ALA ratio (hiLA/loALA diet, ≫5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as it is typical for Western diets. The variations in LA and ALA intake between the probands were minimal in both intervention periods. Moreover, no differences in the (extremely low) background intake of the LC PUFAs AA, EPA, DPAn3 and DHA were observed between the two intervention periods. Total PUFA intake was significantly higher in the loLA/hiALA diet compared to the hiLA/loALA diet due to the higher PUFA content of linseed oil (69.8% of total fatty acids) compared to sunflower oil (62.5% of total fatty acids). The higher MUFA intake in the hiLA/loALA diet was possibly the result of a higher MUFA content of sunflower oil (28.2% of total fatty acids) as compared to linseed oil (19.3% of total fatty acids). The intake of main nutrients (protein, carbohydrates, fat) and energy remained constant between the two intervention periods. These results suggest the applicability of our experimental design and the good compliance of the probands.
1) as it is typical for Western diets. The variations in LA and ALA intake between the probands were minimal in both intervention periods. Moreover, no differences in the (extremely low) background intake of the LC PUFAs AA, EPA, DPAn3 and DHA were observed between the two intervention periods. Total PUFA intake was significantly higher in the loLA/hiALA diet compared to the hiLA/loALA diet due to the higher PUFA content of linseed oil (69.8% of total fatty acids) compared to sunflower oil (62.5% of total fatty acids). The higher MUFA intake in the hiLA/loALA diet was possibly the result of a higher MUFA content of sunflower oil (28.2% of total fatty acids) as compared to linseed oil (19.3% of total fatty acids). The intake of main nutrients (protein, carbohydrates, fat) and energy remained constant between the two intervention periods. These results suggest the applicability of our experimental design and the good compliance of the probands.
RBC fatty acid concentrations
With a few minor exceptions, no significant differences regarding the RBC fatty acid concentrations were observed between the baseline time points of both intervention phases revealing that the run-in and wash-out phase was sufficiently long.The loLA/hiALA diet was effective in increasing ALA and EPA concentrations in RBC membranes. This is in line with the observations of the systematic review of Wood et al.26 who state that a combination of a decrease of LA and a simultaneous increase of ALA intake is most effective in improving the n3 PUFA status.
As expected, the loLA/hiALA diet resulted in a strong increase of ALA concentrations of 332 ± 40% (day 7) and 354 ± 47% (day 14) in RBCs. This comparably large increase of ALA concentrations is greater compared to our previous study, where only ALA was given (12.9 g d−1) via the same linseed oil but without LA restriction.51 In this study the ALA increase in RBCs was 238 ± 24% after 7 days and 294 ± 23% after 3 weeks.51 The study collective was almost identical in both studies. Obviously, in this study the low LA content of the diet (all other dietary factors remained constant) contributed to the greater increase of ALA and n3 PUFAs. The reason is probably a competition between LA and ALA for the incorporation into cell membranes.14
Our finding that EPA concentrations in RBCs significantly increased by 35.0 ± 13% after 7 days and by 57.6 ± 18% after 14 days following the loLA/hiALA diet is a likely result of an increasing conversion of ALA to EPA, since no EPA was ingested via the background diet. Also other studies that increased the total ALA intake to 1.1–6.3 en% observed significantly higher EPA amounts, albeit with great variability27–29,36,38,40–44 reviewed by Wood et al.26 The efficiency of ALA conversion is, besides other factors, mainly dependent on the ALA dose, which essentially explains the fluctuations of the studies cited above.26 In a similar study, a 6-week intervention with ALA from linseed oil (LA/ALA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) resulted in a 47.2% increase of relative EPA amounts in RBCs.33 The reason for this comparatively small increase after 6 weeks of intervention may be the lower ALA dose of 8.7 ± 2.2 g d−1.
1) resulted in a 47.2% increase of relative EPA amounts in RBCs.33 The reason for this comparatively small increase after 6 weeks of intervention may be the lower ALA dose of 8.7 ± 2.2 g d−1.
Furthermore, it is discussed whether the ALA conversion also depends on the LA intake.12,13 Our data support this assumption as the ALA conversion to EPA can be further enhanced by reducing the LA content compared to our previous study with a higher LA intake.51 In our previous study with a similar ALA dose, but uncontrolled and highly variable LA intake (the LA intake was 9.32 ± 5.93 g d−1; 3.2 en%), a smaller increase of EPA concentrations in RBCs was observed (28.5 ± 10% after one week and 49.2 ± 14% after three weeks).51 However, due to the daily contact with the subjects in this study compared to the previous study, a higher level of compliance can be assumed. An influence of the EPA status can be excluded as the relative EPA amount in RBCs was almost the same in the study collective of both studies (0.53 ± 0.04% vs. 0.60 ± 0.04% of total fatty acids of RBC in the previous study). If larger studies confirm this phenomenon, the meaningfulness of recommending a LA/ALA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ref. 19 and 52) without considering absolute LA and ALA intake amounts is questionable. At least the effect of the loLA/hiALA diet on the relative amount of ∑EPA + DHA in RBCs – which is an established marker for protective effects of n3 PUFA with regard to cardiac, cerebral and general health – is stronger compared to a simple ALA supplementation. The relative amount of ∑EPA + DHA in RBCs significantly (p = 0.019) increased by 11.3 ± 3.7% (4.03 ± 0.17% to 4.76 ± 0.20% of total fatty acids) in only 14 days after the loLA/hiALA diet, whereas it remained constant over 12 weeks of daily ALA supplementation without LA restriction.51
1 (ref. 19 and 52) without considering absolute LA and ALA intake amounts is questionable. At least the effect of the loLA/hiALA diet on the relative amount of ∑EPA + DHA in RBCs – which is an established marker for protective effects of n3 PUFA with regard to cardiac, cerebral and general health – is stronger compared to a simple ALA supplementation. The relative amount of ∑EPA + DHA in RBCs significantly (p = 0.019) increased by 11.3 ± 3.7% (4.03 ± 0.17% to 4.76 ± 0.20% of total fatty acids) in only 14 days after the loLA/hiALA diet, whereas it remained constant over 12 weeks of daily ALA supplementation without LA restriction.51
During the hiLA/loALA diet EPA concentrations slightly decreased by −11.2 ± 2% after 7 days and −12.9 ± 3.6% after 14 days. A study with a similar LA/ALA ratio of 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 including 7.8 g d−1 LA (1.4 en%) and 0.30 g d−1 ALA (0.09 en%) also observed decreasing EPA levels in cholesterol ester fatty acids.43 The reason for this EPA decrease may be the fish restricted diet which is lacking EPA and DHA43 and the small amount of ALA available for conversion to EPA. It is likely that the high amount of LA may competitively displace ALA for conversion enzymes.
1 including 7.8 g d−1 LA (1.4 en%) and 0.30 g d−1 ALA (0.09 en%) also observed decreasing EPA levels in cholesterol ester fatty acids.43 The reason for this EPA decrease may be the fish restricted diet which is lacking EPA and DHA43 and the small amount of ALA available for conversion to EPA. It is likely that the high amount of LA may competitively displace ALA for conversion enzymes.
Regarding the effects of ALA supplementation and different LA/ALA ratios on DHA, the results of current studies are heterogeneous. In some studies, DHA in blood remained constant,40 while others found increasing13,53–57 or decreasing51 levels. In the present study, we observed constant DHA concentrations in both intervention phases. Since DHA intake via the background diet was practically unchanged at a very low rate (30 mg vs. 20 mg per day), it is likely that increasing ALA and EPA levels after the loLA/hiALA diet are not converted to DHA. However, the timeframe of 14 days may be too short to observe significant changes in DHA concentrations. Wood et al.26 concluded that ALA supplementation studies with decreased LA intake – as our study – were able to increase DHA concentrations. Our results cannot confirm this observation, on the one hand possibly due to the short intervention time and on the other hand also due to the high ALA dose in this study. The high ALA intake may lead to a competitive saturation of the delta-6 desaturase, and thus the conversion of EPA to DHA (precisely of 24:5n3 to 24:6n3) may be inhibited by the conversion of ALA to EPA (precisely of 18:3n3 to 18:4n3).11 The shift in the ALA/EPA ratio in RBCs from 0.28 ± 0.03 (baseline) to 0.87 ± 0.10 (day 7) and 0.83 ± 0.10 (day 14) indicates that there is indeed a change in the substrate availability for the delta-6 desaturase. Hence, no clear conclusions can be drawn on whether it is possible to improve DHA status without eating fish or other marine products. However, the relative amount of ∑EPA + DHA in RBCs – which is an established marker for health protective effects of n3 PUFA – increased significantly (p = 0.019) in response to the loLA/hiALA from 4.03 ± 0.17% (day 0) to 4.76 ± 0.20% of total fatty acids (day 14). Although the increase is relatively small (and mainly due to the increase in EPA), it would be interesting to investigate the (health) effects on this parameter in an intervention period of more than two weeks.
Only minor changes of the RBC fatty acid patterns were observed in consequence of the hiLA/loALA diet. Concentrations of LA, ALA, AA and DHA were unchanged. Considering that the investigated LA/ALA ratio of 25.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is close to that of the Western diet of 10–20
1 is close to that of the Western diet of 10–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,18,19 the almost constant fatty acid pattern is plausible and therefore in line with the expectations.
1,18,19 the almost constant fatty acid pattern is plausible and therefore in line with the expectations.
Limitations
Despite extensive efforts to create a methodical set-up that allows an adequate examination of the effect of different LA/ALA ratios on the n3 PUFA pattern in blood, this study is also subject to methodical limitations. First, our study is limited by a small sample size and a short duration time, which owes primarily to the extremely elaborate methodology. Additionally, it is questionable if the compliance of the subjects to follow the manifold dietary restrictions to consume a low-fat and low-PUFA diet would have declined with longer study duration. Second, changes in the fatty acid pattern were only measured in RBCs. The changes of PUFA concentrations in RBCs are determined by the blood cell turnover (mean life span of red blood cell is approximately 120 days in circulation) and thus PUFA changes do not fully reach the RBCs. Nevertheless, strong changes in PUFA concentration in RBCs were observed already after seven days suggesting that the PUFA incorporation into newly formed RBCs is sufficient to reflect changes in the PUFA status. Likewise, the fatty acid patterns in RBCs showed the lowest intra-individual variability compared to plasma and plasma phospholipids levels, and thus, appearing as the most suitable biomarker.58Conclusion
We observed a greater increase in RBC EPA concentrations when a high ALA intake was combined with a reduced LA intake (loLA/hiALA diet) compared to a previous study, where we simply increased the ALA intake without LA restriction. Our data support that a high LA intake might impede the ALA conversion to EPA. Further studies are needed to investigate the influence of high LA doses on the n3 PUFA status, especially in view of the high LA and low ALA intake and low ∑EPA + DHA status in many Western countries. Minor changes in the fatty acid profile in consequence of the hiLA/loALA diet suggest that the LA/ALA ratio of 25.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is similar to that of the Western diet.
1 is similar to that of the Western diet.
Abbreviations
| AA | Arachidonic acid |
| ALA | Alpha linolenic acid |
| BMI | Body mass index |
| DHA | Docosahexaenoic acid |
| DPAn3 | n3 docosapentaenoic acid |
| DPAn6 | n6 docosapentaenoic acid |
| en% | Percent of total energy |
| EPA | Eicosapentaenoic acid |
| FAME | Fatty acid methyl ester |
| HDL | High density lipoprotein |
| IS | Internal standard |
| LA | Linoleic acid |
| LC | Long chain |
| LDL | Low density lipoprotein |
| LLOQ | Lower limit of quantification |
| MUFA(s) | Monounsaturated fatty acid(s) |
| n.s. | Not significant |
| n3 | Omega-3 |
| n6 | Omega-6 |
| PUFA(s) | Polyunsaturated fatty acid(s) |
| RBCs | Red blood cells |
| SD | Standard deviation |
| SE | Standard error |
| SFA(s) | Saturated fatty acid(s) |
| TC | Total cholesterol |
| TG | Triglycerides |
Ethics approval and consent to participate
This investigator initiated study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethic committee at the medical chamber of Lower Saxony (Hannover, Germany). Written informed consent was obtained from all subjects.Consent for publication
Not applicable.Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.Funding
This study was supported by grants from the Deutsche Forschungsgemeinschaft (Grant SCHE 1801 and SCHU 2516) to NHS and JPS.Author contributions
The manuscript was written through contributions of all authors. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We would like to thank the participants who contributed their time to this project. We thank Thade Konrad for the help with the analysis of the fat content and the fatty acid profile of food samples.References
- P. C. Calder, Eur. J. Lipid Sci. Technol., 2014, 116, 1280–1300 CrossRef.
- P. C. Calder, Clin. Sci., 2004, 107, 1–11 CrossRef PubMed.
- W. S. Harris, Curr. Atheroscler. Rep., 2009, 11, 411–417 CrossRef PubMed.
- P. E. Marik and J. Varon, Clin. Cardiol., 2009, 32, 365–372 CrossRef PubMed.
- D. Mozaffarian and J. H. Y. Wu, J. Am. Coll. Cardiol., 2011, 58, 2047–2067 CrossRef PubMed.
- T. L. Huang, J. Alzheimers Dis., 2010, 21, 673–690 Search PubMed.
- K. Lukaschek, C. von Schacky, J. Kruse and K.-H. Ladwig, Dementia Geriatr. Cognit. Disord., 2016, 42, 236–245 CrossRef PubMed.
- E. Sydenham, A. D. Dangour and W.-S. Lim, Cochrane Database Syst. Rev., 2012, CD005379 Search PubMed.
- K. D. Stark, M. E. Van Elswyk, M. R. Higgins, C. A. Weatherford and N. Salem, Prog. Lipid Res., 2016, 63, 132–152 CrossRef PubMed.
- B. Lands, Nutrients, 2012, 4, 1338–1357 CrossRef PubMed.
- G. C. Burdge and P. C. Calder, Reprod., Nutr., Dev., 2005, 45, 581–597 CrossRef PubMed.
- J. T. Brenna, Curr. Opin. Clin. Nutr. Metab. Care, 2002, 5, 127–132 CrossRef PubMed.
- G. Burdge, Curr. Opin. Clin. Nutr. Metab. Care, 2004, 7, 137–144 CrossRef PubMed.
- H. Sprecher, D. L. Luthria, B. S. Mohammed and S. P. Baykousheva, J. Lipid Res., 1995, 36, 2471–2477 Search PubMed.
- T. L. Blasbalg, J. R. Hibbeln, C. E. Ramsden, S. F. Majchrzak and R. R. Rawlings, Am. J. Clin. Nutr., 2011, 93, 950–962 CrossRef PubMed.
- B. Lands, Prog. Lipid Res., 2008, 47, 77–106 CrossRef PubMed.
- P. Stehle, Eur. J. Food Res. Rev., 2014, 4, 14 Search PubMed.
- S. Raatz, Z. Conrad, L. Johnson, M. Picklo and L. Jahns, Nutrients, 2017, 9, 438 CrossRef PubMed.
- A. P. Simopoulos, Mol. Neurobiol., 2011, 44, 203–215 CrossRef PubMed.
- K. E. Wood, A. Lau, E. Mantzioris, R. A. Gibson, C. E. Ramsden and B. S. Muhlhausler, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2014, 90, 133–138 CrossRef PubMed.
- A. Patenaude, D. Rodriguez-Leyva, A. L. Edel, E. Dibrov, C. M. C. Dupasquier, J. A. Austria, M. N. Richard, M. N. Chahine, L. J. Malcolmson and G. N. Pierce, Eur. J. Clin. Nutr., 2009, 63, 1123–1129 CrossRef PubMed.
- S. A. Sands, K. J. Reid, S. L. Windsor and W. S. Harris, Lipids, 2005, 40, 343 CrossRef PubMed.
- G. C. Burdge and S. A. Wootton, Br. J. Nutr., 2002, 88, 411 CrossRef PubMed.
- E. Schulte, Dtsch. Lebensmittelrundsch., 2004, 100, 188–189 Search PubMed.
- A. I. Ostermann, M. Müller, I. Willenberg and N. H. Schebb, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2014, 91, 235–241 CrossRef PubMed.
- K. E. Wood, E. Mantzioris, R. A. Gibson, C. E. Ramsden and B. S. Muhlhausler, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2015, 95, 47–55 CrossRef PubMed.
- P. L. Goyens, M. E. Spilker, P. L. Zock, M. B. Katan and R. P. Mensink, Am. J. Clin. Nutr., 2006, 84, 44–53 CrossRef PubMed.
- S. Egert, F. Kannenberg, V. Somoza, H. F. Erbersdobler and U. Wahrburg, J. Nutr., 2009, 139, 861–868 CrossRef PubMed.
- Y. E. Finnegan, A. M. Minihane, E. C. Leigh-Firbank, S. Kew, G. W. Meijer, R. Muggli, P. C. Calder and C. M. Williams, Am. J. Clin. Nutr., 2003, 77, 783–795 CrossRef PubMed.
- P. L. Goyens and R. P. Mensink, J. Nutr., 2005, 135, 2799–2804 CrossRef PubMed.
- L. Hagfors, I. Nilsson, L. Sköldstam and G. Johansson, Nutr. Metab., 2005, 2, 26 CrossRef PubMed.
- M. J. James, V. M. Ursin and L. G. Cleland, Am. J. Clin. Nutr., 2003, 77, 1140–1145 CrossRef PubMed.
- M. D. Kontogianni, A. Vlassopoulos, A. Gatzieva, A.-E. Farmaki, S. Katsiougiannis, D. B. Panagiotakos, N. Kalogeropoulos and F. N. Skopouli, Metabolism, 2013, 62, 686–693 CrossRef PubMed.
- B. A. MacIntosh, C. E. Ramsden, K. R. Faurot, D. Zamora, M. Mangan, J. R. Hibbeln and J. D. Mann, Br. J. Nutr., 2013, 110, 559–568 CrossRef PubMed.
- S. K. Raatz, D. Bibus, W. Thomas and P. Kris-Etherton, J. Nutr., 2001, 131, 231–234 CrossRef PubMed.
- A. L. Wensing, R. P. Mensink and G. Hornstra, Br. J. Nutr., 1999, 82, 183–191 Search PubMed.
- C. Colombo, P. Muti, V. Pala, A. Cavalleri, E. Venturelli, M. Locardi, F. Berrino and G. Secreto, Int. J. Biol. Markers, 2005, 20, 169–176 CrossRef PubMed.
- N. Hussein, E. Ah-Sing, P. Wilkinson, C. Leach, B. A. Griffin and D. J. Millward, J. Lipid Res., 2005, 46, 269–280 CrossRef PubMed.
- D. S. Kelley, G. J. Nelson, J. E. Love, L. B. Branch, P. C. Taylor, P. C. Schmidt, B. E. Mackey and J. M. Iacono, Lipids, 1993, 28, 533–537 CrossRef PubMed.
- D. Li, A. Sinclair, A. Wilson, S. Nakkote, F. Kelly, L. Abedin, N. Mann and A. Turner, Am. J. Clin. Nutr., 1999, 69, 872–882 CrossRef PubMed.
- E. Mantzioris, M. J. James, R. A. Gibson and L. G. Cleland, Am. J. Clin. Nutr., 1994, 59, 1304–1309 CrossRef PubMed.
- A. Y. Taha, Y. Cheon, K. F. Faurot, B. MacIntosh, S. F. Majchrzak-Hong, J. D. Mann, J. R. Hibbeln, A. Ringel and C. E. Ramsden, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2014, 90, 151–157 CrossRef PubMed.
- L. M. Valsta, I. Salminen, A. Aro and M. Mutanen, Eur. J. Clin. Nutr., 1996, 50, 229–235 Search PubMed.
- F. A. Wallace, E. A. Miles and P. C. Calder, Br. J. Nutr., 2003, 89, 679–689 CrossRef PubMed.
- R. J. Pawlosky, J. R. Hibbeln and N. Salem, J. Lipid Res., 2007, 48, 935–943 CrossRef PubMed.
- L. Bakewell, G. C. Burdge and P. C. Calder, Br. J. Nutr., 2006, 96, 93 CrossRef PubMed.
- G. C. Burdge, A. E. Jones and S. A. Wootton, Br. J. Nutr., 2002, 88, 355 CrossRef PubMed.
- F. L. Crowe, C. Murray Skeaff, T. J. Green and A. R. Gray, Br. J. Nutr., 2008, 99, 168–174 CrossRef PubMed.
- E. J. Giltay, L. J. Gooren, A. W. Toorians, M. B. Katan and P. L. Zock, Am. J. Clin. Nutr., 2004, 80, 1167–1174 CrossRef PubMed.
- R. N. Lemaitre, T. Tanaka, W. Tang, A. Manichaikul, M. Foy, E. K. Kabagambe, J. A. Nettleton, I. B. King, L.-C. Weng, S. Bhattacharya, S. Bandinelli, J. C. Bis, S. S. Rich, D. R. Jacobs, A. Cherubini, B. McKnight, S. Liang, X. Gu, K. Rice, C. C. Laurie, T. Lumley, B. L. Browning, B. M. Psaty, Y.-D. I. Chen, Y. Friedlander, L. Djousse, J. H. Y. Wu, D. S. Siscovick, A. G. Uitterlinden, D. K. Arnett, L. Ferrucci, M. Fornage, M. Y. Tsai, D. Mozaffarian and L. M. Steffen, PLoS Genet., 2011, 7, e1002193 CrossRef PubMed.
- T. Greupner, L. Kutzner, F. Nolte, A. Strangmann, H. Kohrs, A. Hahn, N. H. Schebb and J. P. Schuchardt, Food Funct., 2018, 9, 1587–1600 RSC.
- A. P. Simopoulos, Exp. Biol. Med., 2008, 233, 674–688 CrossRef PubMed.
- J. T. Brenna, N. Salem, A. J. Sinclair and S. C. Cunnane, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2009, 80, 85–91 CrossRef PubMed.
- E. A. Emken, R. O. Adlof, H. Rakoff, W. K. Rohwedder and R. M. Gulley, et al. , Biochem. Soc. Trans., 1990, 18, 766–769 CrossRef PubMed.
- M. Plourde and S. C. Cunnane, Appl. Physiol., Nutr., Metab., 2007, 32, 619–634 CrossRef PubMed.
- N. Salem, R. Pawlosky, B. Wegher and J. Hibbeln, Prostaglandins, Leukotrienes Essent. Fatty Acids, 1999, 60, 407–410 CrossRef.
- S. H. Vermunt, R. P. Mensink, M. M. Simonis and G. Hornstra, Lipids, 2000, 35, 137–142 CrossRef PubMed.
- W. S. Harris and R. M. Thomas, Clin. Biochem., 2010, 43, 338–340 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8fo00735g |
| This journal is © The Royal Society of Chemistry 2018 |