Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The short-term supplementation of monacolin K improves the lipid and metabolic patterns of hypertensive and hypercholesterolemic subjects at low cardiovascular risk
Alberto
Mazza
 *a,
Laura
Schiavon
b,
Gianluca
Rigatelli
c,
Gioia
Torin
ad,
Fabio
Montanaro
*a,
Laura
Schiavon
b,
Gianluca
Rigatelli
c,
Gioia
Torin
ad,
Fabio
Montanaro
 e and
Salvatore
Lenti
f
e and
Salvatore
Lenti
f
aESH Excellence Hypertension Centre, Internal Medicine Unit, Santa Maria della Misericordia General Hospital, AULSS 5 Polesana, Rovigo, Italy. E-mail: alberto.mazza@aulss5.veneto.it; Fax: +39 0425 394157; Tel: +39 0425 394567
bDepartment of Medicine, S. Maria della Misericordia General Hospital, AULSS 5 Polesana, Rovigo, Italy
cInterventional Cardiology Unit, Division of Cardiology, S. Maria della Misericordia General Hospital, AULSS 5 Polesana, Rovigo, Italy
dUnit of Internal Medicine C, Department of Medicine, University of Verona, Verona, Italy
eBiostatistics and Data Management Unit, Latis S.r.l., Genova, Italy
fHypertension Centre and Internal Medicine and Geriatrics, San Donato Hospital, USL 8, Arezzo, Italy
First published on 21st June 2018
Abstract
Background – The clinical hypocholesterolemic effect of nutraceutical compounds (NCs) containing red yeast rice extracts providing a daily dose of 2.5–10 mg of monacolin K is now well established. For this reason, NCs may be a viable alternative to the statin drugs commonly used to lower cholesterol levels. However, in order to avoid some possible statin-like side effects, most NCs available on the market contain low doses of monacolin K, which could reduce their efficacy. The aim of this study was to investigate the efficacy and safety of a NC containing high doses of monacolin K (10 mg) in improving the lipid profile and glucose metabolism when added to the diet versus the diet alone in a group of hypertensive and hyper-cholesterolemic subjects at low cardiovascular risk. Methods – Thirty subjects with grade-1 essential hypertension (mean age 51.5 ± 7.8 years, 62.9% males) were enrolled in the treatment group (NC group). These subjects followed a programmed diet and took one tablet a day of a NC containing red yeast rice, policosanols, resveratrol and chromium picolinate for 1 month and were compared with an equivalent group of subjects that followed only a diet program. Differences in serum total cholesterol (TC), low-density- and high-density-lipoprotein cholesterol (LDLC and HDLC), triglycerides (TG) and blood glucose between groups were compared by analysis of variance. Results – In both groups, a significant reduction of TC, TG and LDLC was observed. In the treatment group from the baseline to the follow-up the reduction of TC (230.93 ± 28.0 vs. 188.63 ± 18.1, p < 0.001) and LDLC (153.10 ± 22.5 vs. 116.54 ± 17.7, p < 0.001) was significantly greater compared to the control group (differences between treatments = 9.19% and 12.29%, respectively); in addition a significant higher reduction in blood glucose (89.1 ± 7.6 vs. 83.7 ± 4.6, p < 0.001) was also observed (differences between treatments = 4.28%). HDLC levels remained unchanged in both groups. Conclusions – In summary, the NC containing high doses of monacolin K appeared to be safe, well tolerated and effective at improving lipid and glucose patterns.
Introduction
Hypercholesterolemia is well known to be the main risk factor for cardiovascular disease,1 which represents the most frequent cause of mortality and morbidity in the world. Cardiovascular risk seems to be correlated directly to blood low-density lipoprotein cholesterol (LDLC) levels1 and several epidemiological studies and guidelines have highlighted a relationship between atherosclerosis risk, myocardial infarction and LDLC levels,2,3 hence lowering LDLC levels could reduce cardiovascular risk. The primary preventive approach would be a lifestyle change, with a particular attention to the diet.4–7 However, since it is not easy for everyone to change their life habits, and above all, to maintain a healthy lifestyle for a long time, many hypercholesterolemic subjects need to take hypocholesterolemic drugs. Statins are the drugs of first choice to reduce high cholesterol levels, but in the clinical practice, 10–15% of patients are found to be intolerant to any statin drugs8 and many other refuse them for fear of any adverse events. In fact, statin drugs can cause possible side effects such as muscle pain and damage,9 liver damage,10 diabetes11 and neurological problems.12 Various clinical trials have proved that some nutraceutical blends can provide a benefit, diminishing serum LDLC levels, thus representing a viable natural alternative to statin drugs. In particular, in recent years, research interest has been focusing on the possible lipid-lowering effects of red yeast rice (RYR) supplements,5,13,14 so that their use is recommended in the ESC/EAS guidelines and international documents.3,15,16 RYR is produced by fermentation of white rice by the mold fungus Monacus purpureus that, by its fermenting activity, enriches the rice of a group of substances called monacolines, which possess a marked and scientifically proven hypocholesterolemic activity. Among them, monacolin K (MK), which has a structure identical to levostatin,17 inhibits the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a key enzyme in cholesterol biosynthesis.4,18 Another statin-like property of RYR, containing a fraction of monacolin K, seems to be the cardio-protective effect,19 likely due to a synergistic effect of other products present in the nutraceutical blend.RYR food supplements can vary in the quality and quantity of their components,20 with the daily MK dose ranging from 2.5 to 10 mg. The European Food Safety Authority (EFSA) defined 10 mg of MK as the daily intake dose that effectively contributes to the maintenance of the blood cholesterol level.21 Some case report studies have shown possible side effects related to RYR administration, such as muscle weakness and pain, which tend to subside upon cessation of the treatment,22,23 hepatitis, and symptoms like nausea, vomiting, diarrhea, chills, and daily fever, which improved clinically after RYR discontinuation.24 Currently, most nutraceutical compounds present on the market contain lower doses of MK for fear that a high dose (10 mg) of MK could cause negative side effects similar to those caused by statin drugs. Nutraceutical compounds (NCs) with low doses of MK might be safer, but they are likely less effective.
The aim of the present study was to investigate the efficacy and safety of a NC containing a high dose (10 mg) of MK added to the diet versus the diet alone, in improving the lipid and glucose patterns in a group of hypertensive and hypercholesterolemic subjects at low cardiovascular risk.
Materials and methods
A single site, randomized, open-label, post-market clinical investigation was conducted in accordance with The International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Harmonized Tripartite Guideline for Good Clinical Practice and according to the set of ethical principles laid down in the Declaration of Helsinki. Subjects were consecutively enrolled in our Hypertension Centre after signing and endorsing the informed consent form for the study participation. Enrolled subjects were free to withdraw from the study at any time.The study complied with all institutional and national guidelines, as per the legislative decree no. 211/2013 (Decreto legislativo 24 giugno 2003, n.211). The protocol was approved by the local ethical committee (Comitato Etico dell'Azienda ULSS 5 – Ethical committee of the ULSS 5 Agency) and all participants provided written informed consent.
Inclusion and exclusion criteria
Eligible candidates were men and women aged ≥18 years, with hypocholesterolemia evaluated as the total cholesterol serum level ≥200 mg dl−1 and the LDLC serum level range between 130 and 190 mg dl−1.Exclusion criteria were secondary forms of hypertension, diabetes mellitus, presence of neoplastic or hepatic diseases, chronic heart or renal failure, positive history or clinical signs of ischemic heart disease, disabilities like dementia or inability to cooperate, pregnancy or breast-feeding, use of lipid lowering drugs and organ damage (left ventricular hypertrophy diagnosed by electrocardiogram and carotid plaque by ultrasonography).
Treatment and assessments
The study consisted of a one-month treatment period with two time points for assessments: a baseline examination and a final examination at the end of the treatment.Sixty hypertensive and hypercholesterolemic subjects with grade 1 essential hypertension were enrolled in two study groups. A group was composed of 30 subjects that followed a diet program and took one tablet a day of a nutraceutical blend (Colenorm Plus®) for 1 month. Each tablet of this NC was composed of: 333 mg red yeast rice (10 mg monacolin K), 20 mg policosanols (12 mg octacosanol), 20 mg resveratrol, 50 μg chromium picolinate and 3.15 mg black pepper (2.99 mg piperine) added to the compound to increase the enteric level absorption of resveratrol. The pill count method was used to assess treatment compliance. A control group of 30 subjects with similar characteristics followed only the diet program. The prescribed diet was based on a standardized Mediterranean diet regimen, including a high intake of fish, fruit, vegetables, legumes, olive oil, unrefined whole grains and a moderate intake of lean meats and alcohol.25 The recommended foods to be consumed weekly are summarized in Table 1. Furthermore, the participants had to avoid the consumption of the following foods and ingredients: (1) added sugar (candies, ice cream and table sugar); (2) refined grains (white bread and pasta made with refined wheat); (3) trans fatty acids (i.e. margarine); (4) refined oils (i.e. soybean oil) and (5) processed meat (processed sausages and hot dogs). Participants filled dietary questionnaires and diet regimen compliance was checked by the questionnaire at follow-up (Table 2).
| Foods | 1 Day | 2 Day | 3 Day | 4 Day | 5 Day | 6 Day | 7 Day |
|---|---|---|---|---|---|---|---|
| Cereals | 100 g | 100 g | 100 g | 100 g | 100 g | 100 g | 100 g |
| Vegetables, pasta and olive oils | 200 g fresh salad with olive oil | 150 g pasta with olive oil | 200 g fresh salad with olive oil | 200 g fresh salad with olive oil | 150 g pasta with tomato | 200 g fresh salad with olive oil | 200 g fresh salad with olive oil |
| Yogurt | 150 g | 150 g | 150 g | 150 g | 150 g | 150 g | 150 g |
| Fruit | 150 g fresh | 150 g fresh | 150 g fresh | 150 g fresh | 150 g fresh | 150 g fresh | 150 g fresh |
| Fish and meat | 150 g fish | 100 g meat | 100 g fish | 150 g fish | 100 g meat | 150 g fish | 150 g fish |
| Red wine | 1 glass | 1 glass | 1 glass | 1 glass | 1 glass | 1 glass | 1 glass |
| Eggs | One | — | — | One | — | — | One |
| Cheese | — | 100 g | 100 g | — | 100 g | 100 g | — |
| Foods | All subjects (N = 60) | Control group (N = 30) | Treatment group (N = 30) | p-Values between groups |
|---|---|---|---|---|
| Cereals (%) | 84.0 | 76.7 | 95.0 | 0.087 |
| Vegetables, pasta and olive oils (%) | 96.0 | 96.7 | 95.0 | 0.645 |
| Yogurt (%) | 78.0 | 73.3 | 85.0 | 0.269 |
| Fruit (%) | 92.0 | 90.0 | 95.0 | 0.472 |
| Fish and meat (%) | 74.0 | 76.7 | 70.0 | 0.418 |
| Red wine (%) | 82.0 | 76.7 | 90.0 | 0.207 |
| Eggs (%) | 80.0 | 86.7 | 70.0 | 0.140 |
| Cheese (%) | 94.0 | 93.3 | 95.0 | 0.651 |
During the study period blood samples were taken twice: at the beginning of the study (baseline visit) and at the end of the study (follow-up visit, after one month of treatment), to assess the serum levels of total cholesterol (TC), LDLC and high-density-lipoprotein cholesterol (HDLC), triglycerides (TG), glucose, creatinine (SCr), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatine kinase (CK). Fasting total cholesterol, triglycerides, HDLC, serum glycaemia, creatinine, CK and transaminase levels were measured by the enzymatic method, while LDLC was calculated using Friedewald's formula. Moreover, Body Mass Index (BMI) was calculated as the ratio of weight (in kilograms) to squared height (in meters). In addition, the heart rate (HR), systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured for all study participants. Subjects were classified into current smokers (≥1 cigarette daily) and nonsmokers. The general characteristics of the study group at the baseline are shown in Table 3. BMI, AST, ALT, CK, glycaemia and SCr were evaluated as safety parameters to be monitored. All subjects had normal SCr levels at the baseline (<1.2 mg dl−1 in men and <0.9 mg dl−1 in women).
| All subjects (mean ± SD) (N = 60) | Control group (mean ± SD) (N = 30) | Treatment group (mean ± SD) (N = 30) | p-Value between groups | |
|---|---|---|---|---|
| Age (years) | 52.2 ± 7.9 | 53.0 ± 8.1 | 51.5 ± 7.8 | NS |
| Men (%) | 58.5 | 37.1 | 62.9 | <0.01 |
| Body mass index (kg m−2) | 26.8 ± 4.1 | 27.3 ± 4.8 | 26.3 ± 3.0 | NS |
| Clinic BP values | ||||
| SBP (mmHg) | 141.6 ± 6.0 | 140.1 ± 6.2 | 142.0 ± 5.8 | NS |
| DBP (mmHg) | 85.0 ± 4.8 | 85.8 ± 5.0 | 84.3 ± 4.9 | NS |
| Heart rate (bpm) | 76.5 ± 6.8 | 77.1 ± 6.6 | 76.0 ± 7.0 | NS |
| TC (mg dl−1) | 233.9 ± 22.6 | 236.8 ± 15.6 | 231.0 ± 28.0 | NS |
| LDLC (mg dl−1) | 153.4 ± 18.3 | 153.8 ± 13.2 | 153.1 ± 22.5 | NS |
| HDLC (mg dl−1) | 52.9 ± 13.7 | 55.8 ± 14.7 | 49.9 ± 12.1 | NS |
| TG (mg dl−1) | 137.9 ± 75.2 | 136.5 ± 81.4 | 139.2 ± 69.8 | NS |
| Glucose (mg dl−1) | 91.3 ± 7.5 | 93.0 ± 7.7 | 89.7 ± 7.1 | NS |
| SCr (mg dl−1) | 0.88 ± 0.2 | 0.86 ± 0.2 | 0.90 ± 0.2 | NS |
| AST (U L−1) | 21.1 ± 5.6 | 20.0 ± 4.8 | 22.7 ± 6.3 | NS |
| ALT (U L−1) | 25.6 ± 6.8 | 23.8 ± 4.7 | 27.3 ± 7.5 | NS |
| CK (U L−1) | 89.8 ± 12.0 | 87.4 ± 15.4 | 91.5 ± 11.2 | NS |
| Smokers (%) | 26.7 | 23.3 | 30.0 | NS |
Statistical analyses
Continuous variables were summarized as mean and standard deviation, and compared with analysis of covariance (ANCOVA) and the Bonferroni's post hoc test. Comparison between categorical variables was performed using the χ2 test. Analysis of variance for repeated-measure was used to compare the mean changes of serum lipids, glucose and BMI at the baseline and at the follow-up visit.It was estimated that a sample size of 23 subjects in each group would have had a 90% power to detect a percent difference in means of 15 points in the total cholesterol between the treatment groups at follow-up (i.e., a difference of about 7% between a Group 1 mean, μ1, of 210 and a Group 2 mean, μ2, of 195), assuming a common standard deviation of 15 and using a two group t-test with a 5% two-sided significance level (p < 0.05). Similar estimates were done on other parameters. The number of subjects has been increased to 30 per group (about 20% more) to allow the use of ANCOVA.
All statistical analyses were performed using the SPSS package version 17.0 for Windows (SPSS, Chicago, IL, USA). The null hypothesis was always rejected for values of p < 0.05.
Results
In this study, a group of 30 hypertensive and hypercholesterolemic patients, subjected to both a diet program and the oral administration of a NC with a high dose of MK, was compared to a control group composed of 30 hypertensive and hypercholesterolemic subjects that followed only a diet program. Weekly dietary intake data within both treatment groups, collected through the answers to the dietary questionnaire, are shown in Table 2.Concerning patient's characteristics, no difference was found in the age distribution of the two groups, while a significant difference was found in the percentage of men, higher in the treatment group than in the control group (62.9% vs. 37.1%; p < 0.01) (Table 3).
At the baseline, several parameters were measured in serum: TC, LDLC, HDLC, TG, AST, ALT, CK, glucose and creatinine, besides other subject's characteristics as BMI, SBP, DBP, HR and smoking habits. No significant difference was found at the baseline between the treatment group and the control group for the above listed parameters.
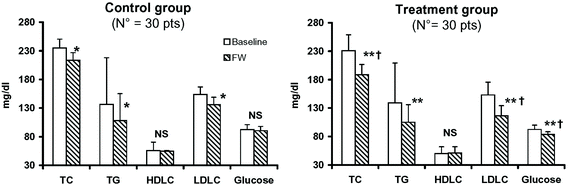
At the end of the treatment, one month after the baseline, all parameters chosen for this study were re-evaluated. In the control group a significant reduction was found between the baseline and follow-up levels of TC, TG and LDLC (respectively: −9.13%; −20.82%; −11.59%; all p < 0.05). No statistical difference was found for HDLC and glucose (Table 4 and Fig. 1A).
| Control group | Treatment group | p-Value between groups | |||||
|---|---|---|---|---|---|---|---|
| Basal (mean ± SD) (N = 30) | Follow-up (mean ± SD) (N = 30) | p-Value | Basal (mean ± SD) (N = 30) | Follow-up (mean ± SD) (N = 30) | p-Value | ||
| TC (mg dl−1) | 234.80 ± 15.6 | 213.37 ± 13.43 | <0.05 | 230.93 ± 28.00 | 188.63 ± 18.10 | <0.001 | <0.0001 |
| TG (mg dl−1) | 136.53 ± 81.42 | 108.10 ± 47.16 | <0.05 | 139.23 ± 69.85 | 105.10 ± 30.84 | <0.001 | NS |
| HDLC (mg dl−1) | 55.77 ± 14.74 | 54.63 ± 1.50 | NS | 49.97 ± 12.01 | 51.07 ± 11.10 | NS | NS |
| LDLC (mg dl−1) | 153.72 ± 13.12 | 135.90 ± 13.00 | <0.05 | 153.10 ± 22.50 | 116.54 ± 17.68 | <0.001 | <0.0001 |
| Glucose (mg dl−1) | 92.37 ± 8.76 | 90.70 ± 7.30 | NS | 89.13 ± 7.64 | 83.70 ± 4.66 | <0.001 | <0.0001 |
In the treatment group, the reduction between the baseline and follow-up for the lipidic profile parameters was greater than in the control group (TC: −18.32%; TG: −24.51%; LDLC: −23.88%; all p < 0.001). Moreover, a significant reduction was found also for glucose levels (reduction of −6.09%; p < 0.001) while no difference was found for HDLC values (Table 4 and Fig. 1B).
Statistical differences emerged between groups (treatment vs. the control group) for TC, LDLC and glucose (differences between treatments = 9.19%, 12.29% and 4.28%, respectively; all p < 0.0001). These data show a significantly greater reduction of TC, LDLC and glucose in the treatment group when compared to the control group, after one month of treatment. However, no differences were found for TG and HDLC values (Table 4 and Fig. 1B).
To assess the safety of the product, safety parameters (AST, ALT, CK, and SCr) were monitored in both groups; the physical well-being was monitored by measuring BMI and blood glucose levels. In the control group, a significant reduction in BMI was found between the baseline and follow-up (27.3 ± 4.8 vs. 26.7 ± 4.6; p < 0.0001). In the treatment group a significant reduction was also found between the baseline and follow-up BMI (26.3 ± 3.0 vs. 25.7 ± 2.3; p < 0.0001), furthermore the glycemic level was also lower at follow up (89.7 ± 7.1 vs. 84.7 ± 4.6; p < 0.0001). No differences were found for the other parameters considered and no differences were found between groups (Table 5).
| Control group | Treatment group | P-Value between groups | |||||
|---|---|---|---|---|---|---|---|
| Basal (mean ± SD) (N = 30) | Follow-up (mean ± SD) (N = 30) | p-Value | Basal (mean ± SD) (N = 30) | Follow-up (mean ± SD) (N = 30) | p-Value | ||
| BMI (kg m−2) | 27.3 ± 4.8 | 26.7 ± 4.6 | <0.0001 | 26.3 ± 3.0 | 25.7 ± 2.3 | <0.0001 | NS |
| Glycemia (mg dl−1) | 93.0 ± 7.7 | 90.7 ± 7.3 | NS | 89.7 ± 7.1 | 84.7 ± 4.6 | <0.0001 | NS |
| SCr (mg dl−1) | 0.86 ± 0.2 | 0.86 ± 0.3 | NS | 0.90 ± 0.2 | 0.90 ± 0.1 | NS | NS |
| AST (U L−1) | 20.0 ± 4.8 | 28.4 ± 6.7 | NS | 22.7 ± 6.3 | 24.3 ± 6.0 | NS | NS |
| ALT (U L−1) | 23.8 ± 4.7 | 27.3 ± 9.2 | NS | 27.3 ± 7.5 | 31.3 ± 6.8 | NS | NS |
| CK (mU mL−1) | 87.4 ± 15.4 | 85.4 ± 16.2 | NS | 91.5 ± 11.2 | 97.0 ± 14.5 | NS | NS |
No adverse event was observed during the study.
Discussion
The present single-site, randomized, open-label, post-market study gives evidence that in hypertensive and hypercholesterolemic subjects with low cardiovascular risk, the supplementation of a NC to the diet results in an improved lipid serum profile and glucose pattern. The main result that emerged from this study is that the NC with a high dose of monacolin K (10 mg) used was safe and well tolerated.Nutraceutical combinations based on RYR may be a valid alternative to statin drugs in hypercholesterolemic subjects with a history of statin drugs’ adverse effects,26,27 and some studies have proved the efficacy of RYR treatment in statin intolerant patients.5,15 Since NCs are not classified as drugs, red rice supplements were not always subjected to strict adverse event monitoring programs. Therefore, attention is increasing for potential risk factors related to the use of nutraceuticals to ensure safety. In fact, the safety of the raw material, the presence of allergenic compounds, the use of vegetable or animal foodstuffs, and the absence of toxicology are parameters to watch out for.28 Several studies focused in proving the efficacy and safety of NCs based on RYR.29–32 However, products containing RYR can cause adverse effects,22 therefore, in order to limit them, before taking any nutraceutical, several factors must be considered, such as the components contained in the blend and their dosage, the dosage of RYR and the amount of MK. MK is the most important molecule among monacolins produced by the fermentation of red rice by the yeast Monacus purpureus; it has a molecular structure similar to lovostatin,16 the precursor of an entire class of statin drugs considered the elective treatment of dyslipidemia and cholesterol control. The ability of monacolin K to act on cholesterol biosynthesis by reducing the function of HMG-CoA reductase as a competitive inhibitor is now known, and many studies have been conducted on several NCs containing RYR with different dosages of monacolin K to study its efficacy and/or safety in hypercholesterolemic subjects. Although monascin and ankaflavin are more potent hypolipidemic agents than monacolin K,33 as far as we know, currently there are no nutraceutical compounds containing monascin and ankaflavin available on the Italian nutraceutical market. On the other hand, our research group has experience with monacolin K at the concentration of 3 mg,34 therefore, we wanted to broaden our experience by testing monocolin K at higher doses (i.e. 10 mg).
The NC tested in this study, is composed, other than RYR, of policosanols, resveratrol, and chromium picolinate. Policosanols (the most important component of which is octacosanol) are natural substances contained in beeswax, potatoes, rice bran and sugarcane, with high hypocholesterolemic activity;35 resveratrol is a polyphenol of vegetable origin mainly extracted from the roots of Polygonum cuspidatum with antioxidant and cardiovascular properties;36,37 while chromium picolinate is able to decrease blood glucose, insulin resistance and lipids in the blood.38,39 According to a previous observational clinical study, but conducted in people with mild/moderate dyslipidemia, resveratrol had a positive effect on the metabolic profile and improved the lipid profile with the absence of side effects.40 Additionally, while synthetic pharmaceuticals are based on single chemicals, many nutraceutical compounds exert their beneficial effects through the additive or synergistic interactions among the different chemical compounds acting at single or multiple target sites associated with a physiological process. In our study, regarding the interactions among the different components of Colenorm Plus, monacolin K, policosanols and resveratrol may interact during lipid metabolism through different mechanisms. In detail, MK may act on cholesterol biosynthesis by reducing the HMG-CoA reductase activity as a competitive inhibitor, whereas policosanols may inhibit intestinal cholesterol absorption and increase the fatty acid β-oxidation. On the other hand, resveratrol may increase the LDLC receptor binding activity and gene expression, suggesting that red wine polyphenols may regulate the major pathways involved in lipoprotein metabolism.
The present study was conducted on a NC characterized by a higher dose of MK (10 mg), showing that a daily intake of 10 mg of MK, added to a diet program, reduced significantly the TC levels by 9.19%, the LDLC levels by 12.29% and the glucose level by 4.28%, compared to subjects that only followed the diet, after one month of treatment. The safety was also evaluated measuring AST, ALT, CK and SCr levels before and after the treatment in both groups, demonstrating the good tolerability of the NC, without any intolerance and adverse reactions. Moreover, this study has demonstrated that one month of diet is enough to reduce BMI, while glycemic levels can be lowered only by the diet combined with the treatment. In particular, our study confirmed the role of Mediterranean diet in reducing the surrogates of the cardiovascular disease such as lipids and glucose levels, highlighting the potential for the Mediterranean diet to act as a key player in cardiovascular disease prevention.3
The results obtained with the NC tested have certainly highlighted its hypocholesterolemic activity, which is a valid alternative to statins and a valuable support to the change in dietary lifestyle. Future results obtained under the same conditions and with the same study design will give us more sound information.
Considering the data obtained in our studies and the data already present in the literature, at this stage it is not yet possible to draw a conclusion on the correlation between the MK dosage and the improvement of the lipid pattern, because most studies don't provide the daily MK dose but only indicate the RYR dosage. Equal amounts of RYR can provide different amounts of MK, and then, to correctly understand the hypocholesterolemic effect of MK, it is crucial to know the quantitative of MK effectively contained in RYR food supplements.41 Commercial RYR products have in fact variable amounts of total monacolin and monacolin K.42 Moreover, to detect the efficacy of the commercial product, the other components of the nutraceutical should be considered, as they can have an additional hypocholesterolemic effect.
Our study has some limitations. The first limitation is the short duration of the treatment period that was chosen to detect the short-term effect of the tested NC. The other limitation is that this is a single site study conducted on a limited population.
On the other hand, the study has also many strengths. In fact, we have chosen a randomized and controlled study design, even if open label, and the amount of monacolin K was determined. Moreover, particular attention was put in detecting any adverse effects, and then, the fact that no adverse event was detected, highlights the safety of the product.
Conclusions
The data emerging from this study showed that a nutraceutical compound, containing 10 mg of MK, appears to be safe and able to improve the lipid serum profile and glucose pattern in hypertensive and hypercholesterolemic subjects. These results need to be confirmed by further studies, by recruiting more subjects and verifying the effect of the NC in a long-term study.List of abbreviations
| ALT | Alanine aminotransferase |
| ANCOVA | Analysis of covariance |
| AST | Aspartate aminotransferase |
| CK | Creatine kinase |
| BMI | Body mass index |
| DBP | Diastolic blood pressure |
| EFSA | European food safety authority |
| HDLC | High-density-lipoprotein cholesterol |
| HR | Heart rate |
| LDLC | Low-density-lipoprotein cholesterol |
| MK | Monacolin K |
| NC | Nutraceutical compound |
| RYR | Red yeast rice |
| SBP | Systolic blood pressure |
| SCr | Serum creatinine |
| SD | Standard deviation |
| TC | Total cholesterol |
| TG | Triglycerides |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
No contributors, other than co-authors, have to be acknowledged. The study was not funded.Notes and references
- F. J. Félix-Redondo, M. Grau and D. Fernández-Bergés, Cholesterol and Cardiovascular Disease in the Elderly. Facts and Gaps, Aging Dis., 2013, 4, 154–169 Search PubMed.
- A. L. Gould, G. M. Davies and E. Alemao, et al., Cholesterol reduction yields clinical benefits: meta-analysis including recent trials, Clin. Ther., 2007, 29, 778–94 CrossRef PubMed.
- A. L. Catapano, I. Graham and G. De Backer, et al., 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias, Eur. Heart J., 2016, 37, 2999–3058 CrossRef PubMed.
- W. B. Borden, Red yeast rice for dyslipidemia in statin-intolerant patients, Curr. Atheroscler. Rep., 2010, 12, 11–13 CrossRef PubMed.
- D. J. Becker, R. Y. Gordon, S. C. Halbert, B. French, P. B. Morris and D. J. Rader, Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial, Ann. Intern. Med., 2009, 150, 830–839 CrossRef PubMed.
- D. J. Becker, R. Y. Gordon, P. B. Morris, J. Yorko, Y. J. Gordon, M. Li and N. Iqbal, Simvastatin vs therapeutic lifestyle changes and supplements: randomized primary prevention trial, Mayo Clin. Proc., 2008, 83, 758–764 CrossRef PubMed.
- L. Hooper, C. D. Summerbell, R. Thompson, D. Sills, F. G. Roberts, H. Moore and G. D. Smith, Reduced or modified dietary fat for preventing cardiovascular, Cochrane Database Syst. Rev., 2011, 7, CD002137 Search PubMed.
- T. R. Joy and R. A. Hegele, Narrative review: statin-related myopathy, Ann. Intern. Med., 2009, 150, 858–868 CrossRef PubMed.
- E. Bruckert, G. Hayem, S. Dejager, C. Yau and B. Bégaud, Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study, Cardiovasc. Drugs Ther., 2005, 19, 403–414 CrossRef PubMed.
- M. W. Russo, M. Scobey and H. L. Bonkovsky, Drug-induced liver injury associated with statins, Semin. Liver Dis., 2009, 29, 412–422 CrossRef PubMed.
- N. Sattar, D. Preiss, H. M. Murray, P. Welsh, B. M. Buckley, A. J. de Craen, S. R. Seshasai, J. J. McMurray, D. J. Freeman, J. W. Jukema, P. W. Macfarlane, C. J. Packard, D. J. Stott, R. G. Westendorp, J. Shepherd, B. R. Davis, S. L. Pressel, R. Marchioli, R. M. Marfisi, A. P. Maggioni, L. Tavazzi, G. Tognoni, J. Kjekshus, T. R. Pedersen, T. J. Cook, A. M. Gotto, M. B. Clearfield, J. R. Downs, H. Nakamura, Y. Ohashi, K. Mizuno, K. K. Ray and I. Ford, Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials, Lancet, 2010, 375, 735–742 CrossRef.
- B. L. Strom, R. Schinnar, J. Karlawish, S. Hennessy, V. Teal and W. B. Bilker, Statin Therapy and Risk of Acute Memory Impairment, JAMA Intern. Med., 2015, 175, 1399–405 CrossRef PubMed.
- V. Verhoeven, M. L. Hartmann, R. Remmen, J. Wens, S. Apers and P. Van Royen, Red yeast rice lowers cholesterol in physicians - a double blind, placebo controlled randomized trial, Complementary Altern. Med., 2013, 13, 178 CrossRef PubMed.
- T. Heinz, J. P. Schuchardt, K. Möller, P. Hadji and A. Hahn, Low daily dose of 3 mg monacolin K from RYR reduces the concentration of LDL-C in a randomized, placebo-controlled intervention, Nutr. Res., 2016, 36, 1162–1170 CrossRef PubMed.
- M. Pirro, C. Vetrani, C. Bianchi and M. R. Mannarino, et al., Joint position statement on Nutraceuticals for the treatment of hypercholesterolemia of the Italian Society of Diabetology (SID) and of the Italian Society for the Study of Arteriosclerosis (SISA), Nutr. Metab. Cardiovasc. Dis., 2017, 27(1), 2–17 CrossRef PubMed.
- A. F. G. Cicero, A. Colletti and G. Bajraktari, et al., Guidelines/recommendations: Lipid lowering nutraceuticals in clinical practice: position paper from an International Lipid Expert Panel, Arch. Med. Sci., 2017, 13(5), 965–1005 CrossRef PubMed.
- M. Y. Hong, N. P. Seeram and Y. Zhang, et al., Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells, J. Nutr. Biochem., 2008, 7, 448–458 CrossRef PubMed.
- Z. Lu, W. Kou, B. Du, Y. Wu, S. Zhao, O. A. Brusco, J. M. Morgan, D. M. Capuzzi, Chinese Coronary Secondary Prevention Study Group and S. Li, Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction, Am. J. Cardiol., 2008, 101, 1689–1693 CrossRef PubMed.
- H. T. Ong and J. S. Cheah, Statin alternatives or just placebo: an objective review of omega-3, red yeast rice and garlic in cardiovascular therapeutics, Chin. Med. J., 2008, 121, 1588–1594 Search PubMed.
- R. Y. Gordon, T. Cooperman, W. Obermeyer and D. J. Becker, Marked variability of monacolin levels in commercial red yeast rice products: buyer beware!, Arch. Int. Med., 2010, 170, 722–1777 Search PubMed.
- European Food Safety Authority (EFSA), Scientific opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL-cholesterol concentrations (ID 1648, 1700) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA panel on dietetic products, nutrition and allergies (NDA), EFSA J., 2011, 9, 2304 Search PubMed.
- P. S. Mueller, Symptomatic myopathy due to red yeast rice, Ann. Intern. Med., 2006, 145, 474–475 CrossRef PubMed.
- D. J. Smith and K. E. Olive, Chinese red rice-induced myopathy, South. Med. J., 2003, 96, 1265–1267 CrossRef PubMed.
- H. Roselle, A. Ekatan, J. Tzeng, M. Sapienza and J. Kocher, Symptomatic hepatitis associated with the use of herbal red yeast rice, Ann. Intern. Med., 2009, 149, 516–517 CrossRef.
- R. Estruch, E. Ros and M. A. Martinez-Gonzalez, Mediterranean diet for primary prevention of cardiovascular disease, N. Engl. J. Med., 2013, 69, 676–677 Search PubMed.
- P. Sikka, S. Kapoor, V. K. Bindra, M. Sharma, P. Vishwakarma and K. K. Saxena, Statin intolerance: now a solved problem, J. Postgrad. Med., 2011, 57, 321–328 CrossRef PubMed.
- K. M. Reinhart and J. A. Woods, Strategies to preserve the use of statins in patients with previous muscular adverse effects, Am. J. Health-Syst. Pharm., 2012, 69, 291–300 CrossRef PubMed.
- A. A. Yates, J. W. Erdman Jr., A. Shao, L. C. Dolan and J. C. Griffiths, Bioactive nutrients - Time for tolerable upper intake levels to address safety, Regul. Toxicol. Pharmacol., 2017, 84, 94–101 CrossRef PubMed.
- S. Gonnelli, C. Caffarelli, K. Stolakis, C. Cuda, N. Giordano and R. Nuti, Efficacy and Tolerability of a Nutraceutical Combination (Red Yeast Rice, Policosanols, and Berberine) in Patients with Low-Moderate Risk Hypercholesterolemia: A Double-Blind, Placebo-Controlled Study, Curr. Ther. Res. Clin. Exp., 2015, 77, 1–6 CrossRef PubMed.
- Y. Li, L. Jiang, Z. Jia, W. Xin, S. Yang, Q. Yang and L. Wang, A Meta-Analysis of Red Yeast Rice: An Effective and Relatively Safe Alternative Approach for Dyslipidemia, PLoS One, 2014, 9, e98611 CrossRef PubMed.
- M. Karl, M. Rubenstein, C. Rudnick and J. Brejda, A multicenter study of nutraceutical drinks for cholesterol (evaluating effectiveness and tolerability), J. Clin. Lipidol., 2012, 6, 150–158 CrossRef PubMed.
- T. C. L. Lin and L. Ming-May, Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia Cheng-Chieh, Eur. J. Endocrinol., 2005, 153, 679–686 CrossRef PubMed.
- A. Santini and E. Novellino, Nutraceuticals in hypercholesterolaemia: an overview, Br. J. Pharmacol., 2017, 174, 1450–1463 CrossRef PubMed.
- A. Mazza, S. Lenti, L. Schiavon, M. Zuin, M. D'Avino, E. Ramazzina and E. Casiglia, Nutraceuticals for Serum Lipid and Blood Pressure Control in Hypertensive and Hypercholesterolemic Subjects at Low Cardiovascular Risk, Adv. Thermoelectr., 2015, 32, 680–690 CrossRef PubMed.
- I. Gouni-Berthold and H. K. Berthold, Policosanol: clinical pharmacology and therapeutic significance of a new lipid-lowering agent, Am. Heart J., 2002, 143, 356–365 CrossRef PubMed.
- D. K. Das and N. Maulik, Resveratrol in cardioprotection: a therapeutic promise of alternative medicine, Mol. Interventions, 2006, 6, 36–47 CrossRef PubMed.
- S. S. Leonard, C. Xia, B. H. Jiang, B. Stinefelt, H. Klandorf, G. K. Harris and X. Shi, Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses, Biochem. Biophys. Res. Commun., 2003, 309, 1017–1026 CrossRef PubMed.
- M. Hummel, E. Standl and O. Schnell, Chromium in metabolic and cardiovascular disease, Horm. Metab. Res., 2007, 39, 743–751 CrossRef PubMed.
- H. Rabinovitz, A. Friedensohn, A. Leibovitz, G. Gabay, C. Rocas and B. Habot, Effect of chromium supplementation on blood glucose and lipid levels in type 2 diabetes mellitus elderly patients, Int. J. Vitam. Nutr. Res., 2004, 74, 178–182 CrossRef PubMed.
- C. Oggioni, L. Della Guardia, P. Pignatti and H. Cena, Nutraceutica nella terapia delle dislipidemie lievi e moderate, Università di Pavia and Fondazione Salvatore Maugeri, IRCCS, Pavia, 2013 Search PubMed.
- D. Heber, A. Lembertas, Q. Y. Lu, S. Bowerman and V. L. Go, An analysis of nine proprietary Chinese red yeast rice dietary supplements: implications of variability in chemical profile and contents, J. Altern. Complement. Med., 2001, 7, 133–1339 CrossRef PubMed.
- Y. G. Li, F. Zhang, Z. T. Wang and Z. B. Hu, Identification and chemical profiling of monacolins in red yeast rice using high-performance liquid chromatography with photodiode array detector and mass spectrometry, J. Pharm. Biomed. Anal., 2004, 35, 1101–1112 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |