Zein-derived peptides as nanocarriers to increase the water solubility and stability of lutein
Yan
Jiao
ab,
Xiqun
Zheng
 a,
Ying
Chang
a,
Dajing
Li
b,
Xiaohong
Sun
a,
Ying
Chang
a,
Dajing
Li
b,
Xiaohong
Sun
 a and
Xiaolan
Liu
*a
a and
Xiaolan
Liu
*a
aCollege of Food and Biological Engineering, Qiqihar University, Qiqihar 161006, China. E-mail: jiaoyan_3000@126.com; liuxiaolan001@126.com
bInstitute of Farm Products Processing, Jiangsu Academy of Agricultural Sciences, Nanjing, China
First published on 16th January 2018
Abstract
Zein and its derived peptides have been used as nanocarriers for bioactive components. Lutein, as well as other xanthophylls, are characterized by blue light filtering and anti-oxidant properties. However, lutein is unstable and has low water solubility, poor absorption, and low bioavailability. In order to protect lutein from oxidative degradation, and to enhance its solubility and dispersibility, stability and bioactivity, lutein-loaded zein nanoparticles (LLZ-NP) and zein-derived peptide nanoparticles (LLZ-PEP-NP) were prepared by the solvent diffusion method. Compared to LLZ-NP, LLZ-PEP-NP possessed good physicochemical properties, including particle size, polydispersity index, zeta potential, entrapment efficiency and in vitro stability. Specifically, transmission electron microscopy (TEM) images showed that LLZ-PEP-NP had a spherical form with a nanometric size and lutein was efficiently loaded into zein-derived peptides through self-assembly. Dynamic light scattering (DLS) results demonstrated that LLZ-PEP-NP had a narrow size distribution in the range of 200–300 nm and a decreased zeta potential compared to that of LLZ-NP. The lutein entrapment efficiency (EE%) of LLZ-NP and LLZ-PEP-NP was more than 85%, while LUT-PEP-NP showed higher lutein entrapment efficiency because of the better capacity of peptides bound with lutein. Nanoencapsulation of lutein into LLZ-PEP-NP resulted in a significantly higher solubility compared to nanoencapsulation of lutein into LLZ-NP and free lutein. The stabilities of lutein in zein-derived peptide nanoparticles in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were improved. These results suggest that zein-derived peptides have the potential to be used as nanocarriers to enhance the solubility and stability of lutein, which can further improve its bioavailability.
1. Introduction
Lutein, an important functional compound, is mainly present in dark green leafy vegetables and marigold flowers. Lutein products have attracted a great deal of attention in recent years because of their functions in the prevention of chronic diseases such as age-related macular degeneration (AMD) and other ocular diseases, atherosclerosis and skin cancer.1–5 However, lutein is an unstable molecule of conjugated carbon–carbon double bonds which has poor absorption after administration and very low bioavailability, caused by its poor solubility in aqueous media.6 The aqueous solubility and chemical stability of lutein may be improved by some carriers.7 Carriers have been recognized as interesting and promising topical delivery vehicles for lutein.Zein is one of the chief protein fractions of corn gluten meal, which is a major by-product of corn wet milling.8 Zein is a protein with several existing and potential applications in the food and pharmaceutical industries. Studies indicate that zein can be applied as microcapsule and nanodelivery systems for bioactive compounds to improve stability and maintain activity in the food industry. Zein is particularly interesting as a naturally occurring polymer for the synthesis of nanodelivery systems. It is a hydrophobic compound classified as generally recognized as safe (GRAS) as a direct human food ingredient by the Food and Drug Administration (FDA).9
Zein could provide alternative encapsulation carriers for the delivery of functional and nutritional components. Several attempts have been made to synthesize zein nanoparticles with entrapped drugs, antimicrobial agents, and bioactive compounds.10,11 However, zein contains a large proportion of hydrophobic amino acids, such as leucine (19.3–21.1%), alanine (8.3–10.5%), proline (9.0–10.5%), and isoleucine (5.7–6.2%), and hence it has low solubility in aqueous systems and exhibits relatively stable structures.12 Zein particles are difficult to release and digest in a simulated stomach system. These properties of zein limit its use as a nanocarrier for bioactive components.13
In recent years, the development of food-derived protein hydrolysates or peptides with nanodelivery systems has attracted particular attention because of the potential benefits associated with higher activity, low molecular weight, high water solubility, easy absorption and few or no negative side effects. Food-derived peptides from some plant proteins like corn, sunflower, soy, wheat germ, and rape seeds as well as some animal proteins such as milk and egg have been reported to synthesize peptide nanoparticles with entrapped drugs.14–16
However, no efforts have been made to utilize zein-derived peptides for lutein coating in food applications. According to our earlier studies, micromolecular hydrolytic peptides had more hydrophilic groups and a smaller size and exhibited good solubility and bioactivity. As a functional carrier, they have the potential to improve the stability, water dispersibility and bioavailability of hydrophobic active ingredients. If zein-derived peptides are used as nanocarriers for lutein, the solubility and digestion stability of lutein can be improved significantly, and the functional properties of the lutein-loaded peptide nanoparticles will be superior to the lutein-loaded zein nanoparticles.
The aim of this study was to increase the water solubility and stability of lutein using zein and its derived peptides nanoencapsulation. Micromolecular hydrolytic peptides were first prepared, and their structural characterisation was analyzed and compared. After the lutein-loaded nanoparticles were prepared using zein and its derived peptides, their characteristics (micromorphology, size, polydispersibility index (PDI), zeta potential, and entrapment efficiency) and in vitro stability were evaluated. The influence of nanoencapsulation on the solubility of lutein was also investigated.
2. Materials and methods
2.1. Materials
Lutein (98%) and zein were purchased from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China) and Beijing Solarbio Science & Technology Co., Ltd (Beijing, China), respectively. The protease Alcalase was purchased from Novo Nordisk (Bagsvaerd, Denmark). Polysulfone ultrafiltration membranes with a cut-off of 8000 daltons were purchased from Kai Jie Membrane Co., Ltd (Hangzhou, China), and the other reagent chemicals were of analytical grade.2.2. Preparation of zein-derived peptides
Alcalase was used to hydrolyze zein according to a method reported in our earlier studies but with some modifications.17 10.0 g of zein was suspended and homogenized in 100 mL of 50 mmol L−1 Tris–HCl buffer (pH 8.0), and then Alcalase was added and the mixture was hydrolyzed at 60 °C for 120 min in a water bath with constant agitation at enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate (E
substrate (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S) ratios of 1
S) ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (g g−1 protein). After hydrolysis, the reaction was terminated by boiling at 100 °C for 10 min. The hydrolysates were centrifuged at 4000 rpm for 20 min, and the supernatants were filtered using an ultrafiltration membrane with a cut-off of 8000 daltons. The separated peptide medium was then spray-dried using a spray drier (B-290, BUCHI, Switzerland) at a flow rate of 10 mL min−1. The inlet and outlet air temperatures were 120 and 55–60 °C, respectively. The spray-dried peptides were collected and stored at −20 °C until further use.
100 (g g−1 protein). After hydrolysis, the reaction was terminated by boiling at 100 °C for 10 min. The hydrolysates were centrifuged at 4000 rpm for 20 min, and the supernatants were filtered using an ultrafiltration membrane with a cut-off of 8000 daltons. The separated peptide medium was then spray-dried using a spray drier (B-290, BUCHI, Switzerland) at a flow rate of 10 mL min−1. The inlet and outlet air temperatures were 120 and 55–60 °C, respectively. The spray-dried peptides were collected and stored at −20 °C until further use.
2.3. Preparation of LLZ-NP and LLZ-PEP-NP
LLZ-NP were prepared by a liquid–liquid dispersion method, according to the method of Chuacharoen et al.18 but with a slight modification. Briefly, 0.5 g of zein was dissolved in 25 mL ethanol aqueous solution (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (v/v)). Lutein solution was prepared at 0.8 mg mL−1 in 100% ethanol and was added dropwise to the zein solution at a ratio of 1
15 (v/v)). Lutein solution was prepared at 0.8 mg mL−1 in 100% ethanol and was added dropwise to the zein solution at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under magnetic stirring (1000 rpm) at room temperature and was centrifuged at 3000 rpm for 10 min. The supernatant was injected into 25 mL of an aqueous phase of pH 5.0; this was kept for half an hour at 50 °C. The LLZ-NP system was transferred to a rotary evaporator and incubated in a 50 °C water bath. Ethanol was removed under a reduced pressure during the evaporation process. The LLZ-PEP-NP were prepared using the same procedure. The free lutein suspension made with the aqueous phase followed the same protocol which served as a control.
1 under magnetic stirring (1000 rpm) at room temperature and was centrifuged at 3000 rpm for 10 min. The supernatant was injected into 25 mL of an aqueous phase of pH 5.0; this was kept for half an hour at 50 °C. The LLZ-NP system was transferred to a rotary evaporator and incubated in a 50 °C water bath. Ethanol was removed under a reduced pressure during the evaporation process. The LLZ-PEP-NP were prepared using the same procedure. The free lutein suspension made with the aqueous phase followed the same protocol which served as a control.
2.4. Entrapment efficiency (EE) measurement
The encapsulation efficiency of lutein in the nanoparticle samples was calculated as the difference between the total amount of lutein used to prepare the loaded systems and the remaining amount of free lutein in the aqueous medium.19 Free lutein was extracted to determine its amount as follows: 1.0 mL of LLZ-NP or LLZ-PEP-NP and 3 mL of petroleum ether were mixed by vortexing vigorously for 5 min at ambient temperature. The mixed sample was centrifuged at 2000 rpm for 5 min and the supernatant was collected in a tube. This was repeated three times.The supernatant was diluted to 10 mL with petroleum ether. The free amount of lutein was, respectively, assayed for lutein content by HPLC at 445 nm (Agilent Technologies, Germany) as described by Li et al. (2015) (YMC-C30 column).20 A calibration curve was made with solutions of lutein at concentrations from 5 to 100 μg mL−1. Each sample was assayed in triplicate. The entrapment efficiency (EE%) was calculated by using the following formula:
| EE% = (1 − Wfree/Wtotal) × 100% | (1) |
2.5. Particle size, polydispersity index (PDI), and zeta potential analyses
Freshly made LLZ-NP and LLZ-PEP-NP samples were characterized by measuring the average diameter size, PDI, and zeta potential by dynamic light scattering (DLS), using a Malvern Zetasizer Nano ZS (Malvern Instruments Ltd, Worcestershire, U.K.). Before the measurements were taken, samples were prepared at a final concentration of 0.02–0.2 mg mL−1, optimum for the instrument. All measurements were performed in triplicate.2.6. Morphology analysis
The morphology of freshly made LLZ-NP and LLZ-PEP-NP was observed by transmission electron microscopy (TEM). One droplet of the sample was placed on a copper grid and allowed to air dry for 5 min. The grid was then negatively stained with 2 g per 100 g phosphotungstic acid aqueous solution for 1 min. Subsequently, the excess liquid was removed with filter paper. After air drying at room temperature, the sample was observed under the TEM.212.7. Fourier transform infrared spectroscopy (FT-IR) assay
An appropriate amount of KBr was dried under an infrared lamp, mixed with the freeze-dried zein and its derived peptides, LLZ-NP and LLZ-PEP-NP samples, and ground into a plate. The plate was scanned by an infrared spectrometer from 4000 to 500 cm−1. The infrared spectra of zein derived peptides were recorded and analyzed in comparison with zein. Infrared spectra of lutein in LLZ-NP and LLZ-PEP-NP were compared to further verify whether lutein was entrapped in zein and its derived peptides.222.8. Solubility of nanoencapsulated and free lutein
The effects of zein and zein-derived peptides nanoencapsulation on the increased solubility and dispersibility of lutein were investigated.23 To determine the solubility of lutein, both nanoencapsulated and non-nanoencapsulated lutein suspensions were centrifuged at 5000 rpm for 10 min, and the supernatant was filtered through a membrane filter (0.45 μm) in order to remove the remaining insoluble lutein. 1.0 mL of the filtered solution was first mixed with 4.0 mL ethanol and vortexed for 2 min, then the suspension was extracted with 15 mL petroleum ether, and the organic phase containing lutein was measured by HPLC.2.9. Stability evaluation of lutein in nanoliposome during in vitro digestion
In vitro digestion of LLZ-NP and LLZ-PEP-NP was carried out in SGF and SIF separately according to the method of Davidov-Pardo et al.24 but with a slight modification, taking LUT as a control group. 5 mL of LLZ-NP and LLZ-PEP-NP solution was mixed with 25 mL SGF or SIF. The final concentration of LUT present in the suspension was 200 μg mL−1. The suspensions were incubated in a shaking water bath (100 rpm) at 37 °C and sampled after 2, 4, 6, 8, 10 and 12 h; 1.0 mL suspensions were sonicated in the presence of 3 mL ethanol, the samples were centrifuged at 3000 rpm for 10 min and the supernatants were collected. The solutions were subsequently assayed for residual LUT content using the HPLC assay as described above.SGF and SIF were prepared according to the method of Frenzel et al.25 but with a slight modification. SGF was produced by mixing 25 ml of distilled water with a pH value set at 2.0 using hydrochloric acid and 1.0 mL of 0.4% pepsin solution (800–2000 U per mg of protein). SIF contained sodium hydroxide (1.81 g L−1), potassium dihydrogen phosphate (8.09 g L−1), pancreatin (4.76 g L−1), and bile salts (5.16 g L−1).
Before in vitro digestion of LLZ-NP and LLZ-PEP-NP, the SGF and SIF were incubated at 37 °C for preheating in the water bath. An HPLC assay of LUT before and after incubation in SGF and SIF was performed to further evaluate the detailed protective effect of zein and its derived peptides on the degradation rate of LUT. The degradation rate was calculated by using the following equation.
| D = C0 − Ct/C0 × 100% | (2) |
2.10. Statistical data analysis
All measurements were repeated three times. The experimental results were statistically tested for significance (p ≤ 0.05) for analysis of variance using SPSS software. All data were expressed as the mean ± standard deviation (SD).3. Results and discussion
3.1. Physicochemical characterization
A liquid–liquid dispersion method was successfully used to synthesize lutein-loaded zein nanoparticles (LLZ-NP) and zein-derived peptide nanoparticles (LLZ-PEP-NP). The formation of zein and its derived peptide nanoparticles is shown in Fig. 1.Lutein loaded zein nanodispersion resulted in the formation of large nanoparticles due to their hydrophobic interactions and then aggregated, which caused turbidity in zein nanodispersion. However, the lutein loaded peptides were able to produce stable nanodispersions and exhibited no visible particles or aggregates. This finding was most likely related to the solubility of zein and peptides.
LLZ-NP, LLZ-PEP-NP and unloaded nanoparticles were characterized after their preparation (Table 1). The average particle size, PDI, and zeta potential of samples were measured.
| Sample | Z-Average diameter (nm) | Polydispersity index (PDI) | Zeta potential (mV) | EE (%) |
|---|---|---|---|---|
| Zein | 482.2 ± 8.25 | 0.538 ± 0.056 | 43.7 ± 2.56 | — |
| Zein-derived peptide | 278.3 ± 5.36 | 0.823 ± 0.068 | −34.3 ± 2.45 | — |
| LLZ-NP | 398.3 ± 5.42 | 0.559 ± 0.019 | 44.3 ± 2.53 | 85.43 ± 4.33 |
| LLZ-PEP-NP | 297.7 ± 7.55 | 0.458 ± 0.026 | −22.5 ± 1.48 | 90.32 ± 3.56 |
The statistical analysis of the data revealed that there was a significant difference in size, PDI and zeta potential between zein, peptides, LLZ-NP and LLZ-PEP-NP. The average particle sizes of zein and its derived peptide nanoparticles were 482.2 ± 8.25 nm and 278.3 ± 5.36 nm, respectively. While the size of LLZ-NP decreased to 398.3 ± 5.42 nm and that of LLZ-PEP-NP increased to 297.7 ± 7.55 nm for binding with lutein when lutein was loaded into zein and peptides, the size of LLZ-PEP-NP was smaller than the size of LLZ-NP.
The size results were confirmed by TEM (Fig. 2). Zein and its derived peptides showed a spherical shape and the size of zein was larger than that of its derived peptides. When lutein was loaded into zein nanoparticles, the size of nanoparticles decreased and aggregated, which were in agreement with the particle size and nanodispersion profile. Similar zein nanoparticle images have been reported in other studies.26,27 The LLZ-PEP-NP showed an irregular sphere and good dispersibility, and lutein was encapsulated in peptide particles, resulting in a higher entrapment efficiency (Fig. 2A and B).
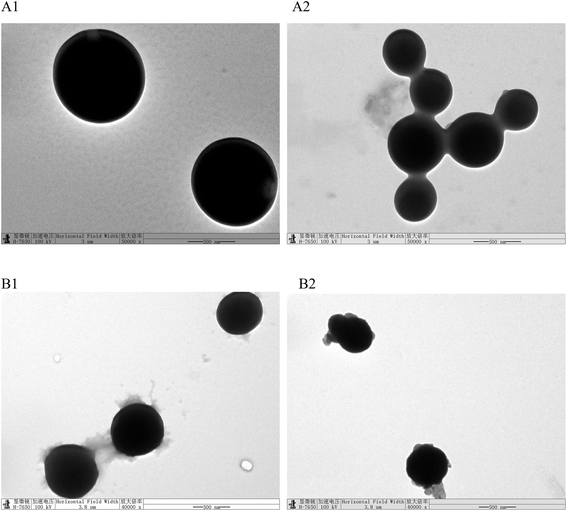 | ||
| Fig. 2 Transmission electron microscopy (TEM) images of zein and zein-derived peptides (A1 and B1), LLZ-NP and LLZ-PEP-NP (A2 and B2). | ||
Zeta potential data showed that zein possessed an average cationic charge in acidic condition. A slight alteration in zeta potential from 43.7 ± 2.56 mV to 44.3 ± 2.53 mV was observed for zein incorporating lutein. Zein derived peptides possessed an average negative charge, and entrapment of lutein resulted in a significant change in zeta potential from −34.3 ± 2.45 mV to −22.5 ± 1.48 mV. The obvious changes of zeta potential revealed that the micromolecular peptide of zein could be coupled with lutein easily through the electrostatic attraction of positively charged peptide chains with the negative regions of the lutein and exhibited higher stability and entrapment efficiency than that of zein.
Meanwhile, the hydrophobic interaction between lutein and zein derived peptides was weaker than that of zein. Thus, the EE of LLZ-NP was around 85.43 ± 4.33%, and the EE of LLZ-PEP-NP was 90.32 ± 3.56, which was higher than that of LLZ-NP.
3.2. FT-IR assay
FTIR spectroscopy was used to analyze the total composition of these nanoparticles. The spectra for zein and zein derived peptides LLZ-NP and LLZ-PEP-NP were identical. Fig. 3A shows the FTIR spectrum of zein and peptides from 4000 to 500 cm−1. This spectrum shows that the predominant component of the hydrolyzed zein is protein and peptides, as characterized by absorption bands such as amide (vibration absorption of N–H at 3405 cm−1 and 3315 cm−1), amide I (1750–1600 cm−1), amide II (1500–1400 cm−1), and hydroxyl (vibration absorption of O–H at 2960 cm−1), and the location of peaks and the form of zein and zein derived peptides were similar.28 When zein was hydrolyzed, strong amide signals were observed, from 3405–3315 cm−1, and an increase of the amide signals could be seen at 1660–1413 cm−1. The increase of the groups in the peptides could enhance the adsorption with lutein for their electrostatic interaction, which is beneficial for embedding lutein and increasing the dispersibility of lutein.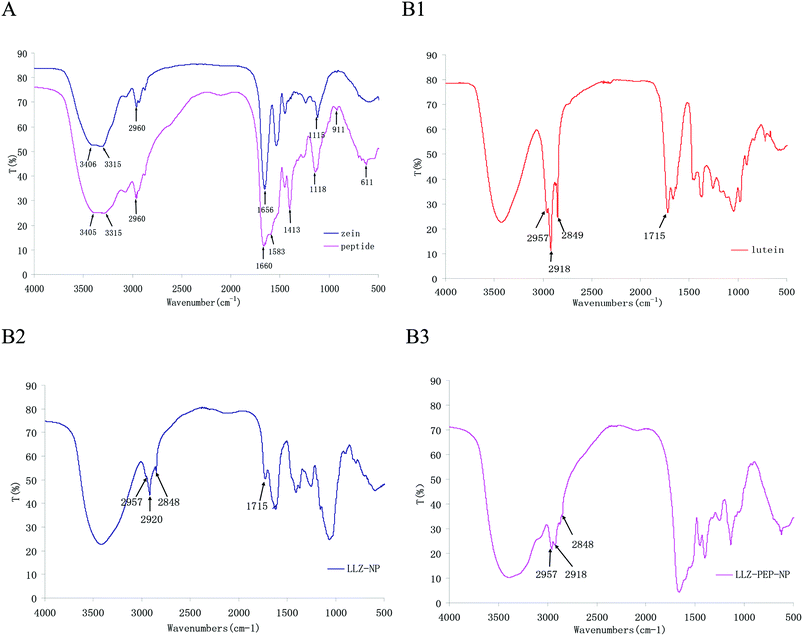 | ||
| Fig. 3 FTIR spectra of zein and zein-derived peptides (A), lutein, LLZ-NP and LLZ-PEP-NP (B1, B2 and B3). | ||
As seen in Fig. 3B(1–3), when lutein was coated with zein and hydrolytic peptides, in the region of wavenumbers 2957 cm−1 (vibration absorption of O–H), 2920 and 2848 cm−1 (vibration absorption of C–H), and 1715 cm−1 (symmetrical stretching vibration absorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), the location of peaks and the form of LLZ-NP and LLZ-PEP-NP were similar, which were characteristic peaks of lutein and indicated the existence of lutein in zein and peptide nanoparticles.29 FT-IR spectra confirmed that lutein had been successfully entrapped by zein and zein derived peptides.
C), the location of peaks and the form of LLZ-NP and LLZ-PEP-NP were similar, which were characteristic peaks of lutein and indicated the existence of lutein in zein and peptide nanoparticles.29 FT-IR spectra confirmed that lutein had been successfully entrapped by zein and zein derived peptides.
3.3. Effect of LLZ-NP and LLZ-PEP-NP on lutein solubility and dispersibility
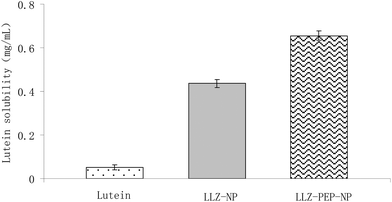
To analyze the capacity of LLZ-NP and LLZ-PEP-NP to aid in the solution and dispersion of lutein, the solubility of nanoencapsulated and free lutein was evaluated. All of the samples for the solubility and dispersibility tests were fixed at 0.8 mg mL−1. As shown in Fig. 4, the soluble and dispersive lutein was significantly increased from 0.051 mg mL−1 to 0.437 mg mL−1 by LLZ-NP nanoencapsulation. This phenomenon was more clearly observed in the LLZ-PEP-NP suspension compared with the free lutein suspension (lutein/distilled water) containing lutein and distilled water. Encapsulation of lutein in zein-derived peptide nanoparticles allows their dissolution and dispersion in water partly, the soluble and dispersive lutein was significantly increased to 0.655 mg mL−1 by zein derived peptides nanoencapsulation. Therefore, the LLZ-PEP-NP suspension showed significantly higher solubility and dispersibility of lutein than the free lutein/distilled water suspension (12.84 times higher). These results can be explained by the inhibition of lutein crystallization due to nanoencapsulation, resulting in decreased molecular mobility of the lutein and thus the improved solubility or dispersibility of the entrapped lutein. In particular, the hydrophilicity of zein peptides improved the solubility of lutein.30 These results suggest that lutein solubility and dispersibility are increased by LLZ-NP and LLZ-PEP-NP and that zein derived peptides play an important role in improving the solubility of lutein.3.4. In vitro digestion stability
The degradation rates of lutein, LLZ-NP and LLZ-PEP-NP in SGF and in SIF were estimated to investigate the digestion stability in vitro, respectively. The remaining content of lutein was used to evaluate the degradation rates of lutein in the nanoliposome, taking lutein as a control group. Fig. 5 shows that a rapid degradation of lutein occurred when incubated in SGF and SIF after 12 h. The free lutein degradation rates rose to 46.15 ± 1.743 and 44.13 ± 1.18 after 12 h, respectively.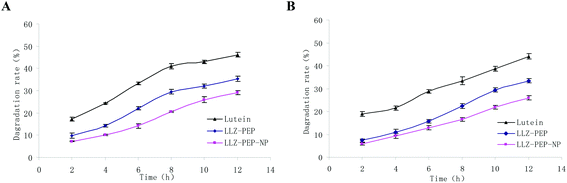 | ||
| Fig. 5 Lutein degradation rate of free lutein, LLZ-NP, and LLZ-PEP-NP during digestion in SGF (A) and in SIF (B) for 12 h. | ||
Degradation of lutein was significantly reduced after encapsulating in LLZ-NP. The degraded lutein in LLZ-NP after 12 h incubation in SGF (Fig. 5A) and SIF (Fig. 5B) was 35.34 ± 1.091% and 33.447 ± 0.908%, respectively.
After encapsulating in LLZ-PEP-NP, the lutein degradation rate dropped to 29.10 ± 0.806% and 25.98 ± 0.932% after 12 h incubation in SGF (Fig. 5A) and in SIF (Fig. 5B). The results evidenced that hydrochloric acid in gastric juice and pepsase could weaken the interaction of lutein with zein and some of the zein hydrolyses under acidic conditions; the lutein degradation rates increased slightly in SGF.
Peptides displayed better stability in SGF and SIF, and they could protect lutein from degradation. The result verified that LLZ-NP and LLZ-PEP-NP could decrease the degradation of lutein in SGF and in SIF. Therefore, it could be concluded that the stability of lutein in SIF could be improved by encapsulation in LLZ-NP and LLZ-PEP-NP. Furthermore, the lutein protective capability of zein-derived peptides was higher than that of zein.
4. Conclusion
Lutein has been studied extensively for its blue light filtering and anti-oxidant properties. However, instability and poor water solubility limit its applications. Zein and zein-derived peptides have already found interesting applications as dietary supplements and pharmaceutical preparations. In this work, zein and zein-derived peptide nanoparticles loaded with lutein were successfully synthesized using a liquid–liquid dispersion method. The result showed that lutein loaded zein resulted in the formation of large nanoparticles due to their hydrophobic interactions which led to aggregation. However, the lutein loaded peptides resulted in the formation of small nanoparticles. They exhibited good dispersibility due to the increased amide groups in peptide which were beneficial for incorporation with lutein. As compared to LLZ-NP, the LLZ-PEP-NP increased the entrapment efficiency of lutein from 85.43% to 90.32%, the solubility of lutein was increased more than 12-fold by nanoencapsulation into zein-derived peptide nanoparticles. The stability of lutein in zein-derived peptides nanoparticles in SGF and SIF was improved.These results suggest that smaller molecule peptides were obtained after limited hydrolysis of zein, which showed superior carrier performance to lutein. Zein-derived peptide nanoencapsulation is a novel and effective delivery system for improving the solubility and stability of lutein and other poorly water soluble compounds, which may be beneficial for future applications in the development of functional foods. Furthermore, LLZ-PEP-NP could enhance lutein performance by improving the solubility and bioavailability, and in vitro and in vivo stability. Our future research involves in vivo absorption evaluations, including cell uptake and cellular antioxidant activity of lutein entrapped in zein-derived peptides which will be further investigated.
Conflicts of interest
The authors declare no conflict of interest concerning the content of this article.Acknowledgements
This research study was supported by the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No. UNPYSCT-2016091) and by the National Natural Science Foundation of China (Grant No. 31371726).References
- A. Sujak, J. Gabrielska, W. Grudziński, R. Borc, P. Mazurek and W. I. Gruszecki, Arch. Biochem. Biophys., 1999, 371, 301–307 CrossRef CAS PubMed.
- E. S. M. Abdel-Aal and I. Rabalski, Am. J. Biomed. Sci., 2013, 5, 109–125 CrossRef CAS.
- N. I. Krinsky, J. T. Landrum and R. A. Bone, Annu. Rev. Nutr., 2003, 23, 171–201 CrossRef CAS PubMed.
- D. C. Musch, JAMA Ophthalmol., 2014, 132, 139–141 CrossRef CAS PubMed.
- S. Y. Li, F. K. Fung, Z. J. Fu, D. Wong, H. H. Chan and A. C. Lo, Invest. Ophthalmol. Visual Sci., 2012, 53, 5976–5984 CAS.
- I. Lacatusu, E. Mitrea, N. Badea, R. Stan, O. Oprea and A. Meghea, J. Funct. Foods, 2013, 5, 1260–1269 CrossRef CAS.
- D. Y. Hong, J. S. Lee and H. G. Lee, Int. J. Biol. Macromol., 2015, 85, 9–15 CrossRef PubMed.
- C. Zhou, J. Hu, X. Yu, A. E. A. Yagoub, Y. Zhang, H. Ma and P. N. Y. Otu, LWT – Food Sci. Technol., 2017, 77, 488–496 CrossRef CAS.
- A. O. Elzoghby, W. M. Samy and N. A. Elgindy, J. Controlled Release, 2012, 161, 38–49 CrossRef CAS PubMed.
- F. Xue, C. Li, Y. Liu, X. Zhu, S. Pan and L. Wang, J. Food Eng., 2013, 119, 439–445 CrossRef CAS.
- Y. P. Wu, Y. G. Luo and Q. Wang, LWT – Food Sci. Technol., 2012, 48, 283–290 CrossRef CAS.
- J. M. Kim, J. H. Whang, K. M. Kim, J. H. Koh and H. J. Suh, Process Biochem., 2004, 39, 989–994 CrossRef CAS.
- T. Chuacharoen and C. M. Sabliov, Colloids Surf., A, 2016, 503, 11–18 CrossRef CAS.
- A. O. Elzoghby, W. M. Samy and N. A. Elgindy, J. Controlled Release, 2012, 161, 38–49 CrossRef CAS PubMed.
- N. A. El-Wakil, E. A. Hassan, R. E. Abou-Zeid and A. Dufresne, Carbohydr. Polym., 2015, 124, 337–346 CrossRef CAS PubMed.
- J. Yi, Y. Fan, W. Yokoyama, Y. Zhang and L. Zhao, Food Chem., 2016, 200, 91–97 CrossRef CAS PubMed.
- X. L. Liu, X. Q. Zheng, Z. L. Song, X. F. Liu, N. Kumar Kopparapu, X. J. Wang and Y. J. Zheng, J. Funct. Foods, 2015, 18, 1147–1157 CrossRef CAS.
- T. Chuacharoen and C. M. Sabliov, Colloids Surf., A, 2016, 503, 11–18 CrossRef CAS.
- C. Prego, D. Torres, E. Fernandezmegia, R. Novoacarballal, E. Quiñoá and M. J. Alonso, J. Controlled Release, 2006, 111, 299–308 CrossRef CAS PubMed.
- D. J. Li, J. F. Song and C. Q. Liu, Int. J. Food Prop., 2015, 18, 178–185 CrossRef CAS.
- C. Tan, B. Feng, X. Zhang, W. Xia and S. Xia, Food Hydrocolloids, 2016, 52, 774–784 CrossRef CAS.
- L. Q. Zou, W. Liu, W. L. Liu, R. H. Liang, T. Li, C. M. Liu, Y. L. Cao, J. Niu and Z. Liu, Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization, J. Agric. Food Chem., 2014, 62, 934–941 CrossRef CAS PubMed.
- G. Spigno, F. Donsì, D. Amendola, M. Sessa, G. Ferrari and D. M. De Faveri, J. Food Eng., 2013, 114, 207–214 CrossRef CAS.
- G. Davidov-Pardo, C. E. Gumus and D. J. Mcclements, Food Chem., 2016, 196, 821–827 CrossRef CAS PubMed.
- M. Frenzel and A. Steffen-Heins, Food Chem., 2015, 173, 1090–1099 CrossRef CAS PubMed.
- N. Parris, P. H. Cooke and K. B. Hicks, J. Agric. Food Chem., 2005, 53, 4788–4792 CrossRef CAS PubMed.
- Y. Zhang, Y. Niu, Y. Luo, M. Ge, T. Yang, L. L. Yu and Q. Wang, Food Chem., 2014, 142, 269–275 CrossRef CAS PubMed.
- T. C. Bicudo, L. A. Forato, L. A. R. Batista and L. A. Colnago, Anal. Bioanal. Chem., 2005, 383, 291–296 CrossRef CAS PubMed.
- G. H. Shin, S. K. Chung, J. T. Kim, H. J. Joung and H. J. Park, J. Agric. Food Chem., 2013, 61, 11119–11126 CrossRef CAS PubMed.
- T. Vasconcelos, B. Sarmento and P. Costa, Drug Discovery Today, 2007, 12, 1068–1075 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |