Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Safety considerations of plant polysaccharides for food use: a case study on phenolic-rich softwood galactoglucomannan extract
Leena
Pitkänen
 *,
Marina
Heinonen
and
Kirsi S.
Mikkonen
*,
Marina
Heinonen
and
Kirsi S.
Mikkonen

Department of Food and Nutrition, P.O. Box 66, FIN-00014, University of Helsinki, Finland. E-mail: leena.m.pitkanen@helsinki.fi
First published on 13th March 2018
Abstract
A growing population and concern over the sufficiency of natural resources for feeding this population have motivated researchers and industries to search for alternative and complementary sources of food ingredients and additives. Numerous plant species and parts of plants are explored as raw materials for food production. An interesting example is wood; to date, only a few wood-based additives or ingredients are authorized for food use. Wood hemicelluloses, such as softwood galactoglucomannans (GGM), constitute an abundant bioresource that shows a high potential functionality in edible materials. Spruce GGM acts as a multi-functional emulsion stabilizer, and it could be used in various processed food products, replacing less effective, conventional emulsifiers. Before new materials can be released into the food market, their safety must be evaluated, according to the Novel Food regulation. This review focuses on the safety aspects that must be considered before polysaccharide- and phenolic-rich plant extracts can be awarded the status of authorized food ingredients. In this review, GGM is presented as a case study and examples are given of plant-based polysaccharides that are already authorized for food purposes. The legislation regarding Novel Food ingredients in Europe is also briefly reviewed.
1. Introduction
Polysaccharides are polymeric carbohydrates that consist of monosaccharide units, which are connected together with glycosidic bonds. Due to the structural variation of different monosaccharides as well as the innumerable ways that these building blocks link with each other, polysaccharides can be considered as structurally complex biomacromolecules. Polysaccharides originating from plants (e.g., starch and guar gum), microbes (e.g., xanthan), algae (e.g., alginates and carrageenans) and animals (e.g., glycogen and chitin) are frequently used in food. Starch, a high molar mass compound consisting of (1→4)-linked α-D-glucopyranosyl units, is an important energy nutrient that is abundant in common foods, such as cereals and root crops. Although many other food polysaccharides are not digested in the upper gastrointestinal tract of humans, they often serve functions other than being components giving nutritional value. For example, plant cell-wall polysaccharides, such as arabinoxylans and β-glucan, exist in cereal-based foods, and “plant gums” are used as thickeners, emulsifiers, emulsion stabilizers, gelling agents and encapsulating agents.1 These non-digestible polysaccharides are important for health because they are considered as dietary fibre, which promote colon health, regulate post-prandial blood glucose levels and reduce serum cholesterol levels.2Despite the fact that nature provides various sources of polysaccharides, and that scientific research on their exploitation as food materials is increasingly active, a relatively low number of polysaccharides are authorized for use as food ingredients. For example, in the European Union (EU) and in Switzerland, among the 334 permitted food additives (identified by an E number) less than 40 are polysaccharide-based (native or structurally modified). The difference between other food ingredients and food additives is mainly the quantity used in any given product. Food ingredients can be consumed alone as food (e.g., starch), whereas food additives (e.g., carboxymethyl cellulose) are used in small quantities (usually less than 2%) relative to the total food composition but they, nonetheless, play an important role in the food products. Regarding food additive use in Europe, the European Food Safety Authority (EFSA) has an expert Panel on Food Additives and Nutrient Sources Added to Food (ANS), which evaluates the safety of food additives. Similarly, if new ingredients are released into the market, EFSA's Panel on Dietetic Products, Nutrition and Allergies (NDA) has the responsibility of evaluating the safety of Novel Food ingredients.
The vast majority of polysaccharides used as food ingredients are plant-based. For example, guar gum (galactomannan) is extracted from the seeds of the leguminous plant Cyamopsis tetragonolobus, and gum arabic is an exudate obtained from the sap of Acacia trees (Acacia seyal or Acacia senegal). Woody tissue offers an attractive source for additives, such as cellulose microcrystals and various cellulose derivatives. In addition to the cellulosic polysaccharides, other types of food-grade ingredients or additives, such as, vanillin aroma, glycerol esters of wood rosins (E445), xylitol (E967) and steryls/stanols, are derived from wood. Despite the fact that these wood-based, food-grade materials are already on the market, wood biomass could be utilized more effectively. The main components of wood are polysaccharides: cellulose (40–50 wt%) and hemicelluloses (20–35%), while lignin comprises 15–30% of wood mass.3 In addition to these macromolecules, wood contains a small amount of inorganic residues and extractives, which are low molar mass molecules. Extractives include a heterogeneous group of aliphatic and cyclic compounds: terpenes and terpenoids, esters of fatty acids, fatty acids, alcohols, alkanes, simple phenols, stilbenes, lignans, isoflavones, condensed tannins, flavonoids and hydrolyzable tannins. Wood phenolic compounds may possess bioactive functions; in vitro studies suggest that they may act as antioxidants.4 Due to the close association of lignin and extractives with cellulose and hemicelluloses, low amounts of these compounds commonly exist in hemicellulose or cellulose extracts and can, thus, be considered as “co-passengers” of fibrous materials. While wood extracts are neither presently nor extensively used in food ingredients, they have a long history in food supplement use. Softwood extracts have also recently received attention in the biomedical field; spruce hemicellulose extract was patented for “use on the treatment of lower urinary tract symptoms and diseases”.5,6
O-Acetyl-galactoglucomannan (GGM) from spruce (Picea abies) is an example of a highly interesting wood-based polysaccharide7 that could be used in food but is not currently in the list of accepted food ingredients, including food additives. Before new compounds can be released into the market in the EU, their safety must be evaluated, as legislated by the European Commission. This review presents a case study of GGM, a new, highly potential hydrocolloid on its route to an authorized food ingredient (Fig. 1). The review explores the safety aspects of phenolic-rich plant polysaccharide extracts with a special focus on GGM extract (in this article the abbreviation “GGM” is used for GGM extract which contains small amounts of other compounds, such as lignin and extractives in addition to galactoglucomannan). Examples from the safety considerations of the polysaccharides that are already permitted for food use are presented. Additionally, the safety aspects and data requirements for safety evaluation as Novel Food or Novel Food ingredients will be discussed.
2. Spruce galactoglucomannan (GGM) – a potential wood-based food ingredient
Hemicelluloses, structural elements of the plant cell-walls, are a heterogeneous group of polysaccharides and constitute almost one third of plant mass. Unlike cellulose, hemicelluloses have a limited number of industrial applications because the methods for isolating and recovering them from lignocellulosic biomasses were previously lacking due to technological and economical limitations. Thus, hemicelluloses constitute a remarkable bioresource that could be utilized more efficiently in the future. Other polysaccharides, such as starch, guar gum, locust bean gum, konjac glucomannan and gum arabic, have significant roles; for example, in food technology, they bind water and increase viscosity (modify and stabilize food structures), form networks and gels and act in emulsions as emulsifiers and emulsion stabilizers (lower the surface tension between water and oil and increase the viscosity of the continuous phase).8 GGM is a highly potential hemicellulosic polysaccharide that could be recovered from softwood and utilized as a Novel Food component. Various studies highlight the applicability of GGM in emulsions,9–11 in edible (and biodegradable) films12–16 and in hydrogels and aerogels.17–19 In addition to GGM, other wood-based hemicelluloses, namely, birch xylans are promising food ingredients; birch pulp xylan improved the structure of low-fat acid milk gel products (yogurts) and acted as a dietary fibre component.20A unique characteristic of GGM is that it acts as a multi-functional emulsion stabilizer, both preventing physical breakdown and inhibiting lipid oxidation. Also, GGM showed surface activity in emulsion studies, i.e., it has a capacity to lower the surface tension of water, which is significant in emulsification.11 As shown in Fig. 2, one month's storage of rapeseed oil-in-water emulsion, with 60 wt% oil and 12% GGM, had no effect on the droplet size distribution, indicating that the emulsion was physically stable. Rheological characterization indicated that the mechanism behind GGM's excellent physical stabilizing capacity is partially related to the steric effect and partially to the viscosity increase of the continuous phase. The viscosity of GGM solutions is between that of small-molecular surfactants and high molar mass macromolecular stabilizers.11 Physical stabilization alone does not guarantee the shelf life of a product, but GGM also efficiently stabilizes emulsions chemically by inhibiting lipid oxidation.10 The oxidation of lipids (especially unsaturated fatty acids) is a significant factor, reducing the quality of food by reducing the nutritional value and by introducing components that bring undesirable flavours to food (i.e., reduce the sensory quality).
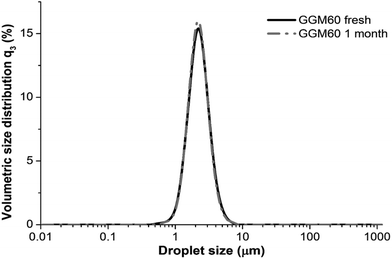 | ||
Fig. 2 GGM is an efficient emulsion (oil-in-water) stabilizer. No change in the droplet size distributions of the fresh emulsion and the emulsion that has been stored for one month at room temperature can be observed. The emulsion contains 60 wt% of oil and the emulsifier-to-oil ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Reprinted from ref. 11, courtesy of the Royal Society of Chemistry. 5. Reprinted from ref. 11, courtesy of the Royal Society of Chemistry. | ||
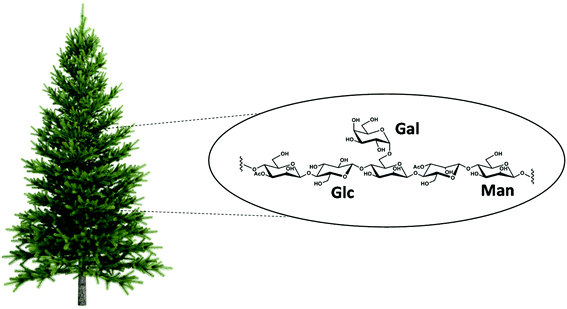
The polysaccharide structure of GGM consists of (1→4)-linked β-D-mannopyranosyl and β-D-glucopyranosyl units with (1→6)-linked galactose side units attached to mannose units (Fig. 3). However, when referring to GGM, we consider the complete extract mixture obtained from wood, in which polysaccharides are clearly the major constituents. Depending on the extraction method used, other (non-polysaccharide) compounds are co-extracted at various amounts. This is also the case for other polysaccharides; for example, galactomannan from guar (guar gum) or arabinogalactan from Acacia trees (gum arabic), which are widely used as food additives, are known to contain small amounts of protein.8,21
Spruce GGM can be extracted in polymeric form using different procedures—the molar mass of GGM varies between a few thousand g mol−1 and a few tens of thousands g mol−1, depending on the extraction method and analysis technique employed for molar mass determination. Classically, hemicelluloses can be extracted from plant tissues using basic extraction solutions (e.g., NaOH and KOH), but acetyl groups, which promote the water-solubility of hemicelluloses at intermediate degrees of acetylation, are cleaved during the alkaline treatment of the plant material. The environmentally friendly way of recovering GGM from a wood matrix is a pressurized hot water extraction (PHWE) method.22 Also, a related extraction method, utilizing water as the extraction solvent, has recently been developed for biomass fractionation.23 During the extraction process, the extractant is circulated through the fibre matrix to remove the impurities from the extract. Another way to extract GGM, which carries acetyl groups, is to recover the polysaccharide from the process water of the thermomechanical pulping (TMP) process.24
Due to recent developments in large-scale extraction techniques,22 GGM is available in kilogram quantities. Kilpeläinen et al. successfully scaled PHWE from a laboratory scale to a pilot scale, which increased the extraction capacity by a factor of 6000. PHWE utilizes plain (tap) water as a solvent and no other chemicals are used. Water can also be recycled after extractions. Thus, the extraction process is environmentally friendly. In pilot scale PHWE, water is pumped through the extraction vessel (volume 300 l), which is filled with spruce sawdust (particle size distributing between 0.2 and 4 mm), for extraction of GGM. The extraction was performed at 170 °C for 60 min. Sawdust was pre-steamed to heat the sawdust inside the extraction vessel evenly and to decrease the channelling of water flowing through the sawdust bed. The extraction power of PHWE is based on a decrease of the dielectric constant of water at subcritical temperatures, and thus, water can dissolve more semi-polar compounds. At high temperatures, the viscosity of water is also lower than that at room temperature, which enhances the penetration capability of water into the sample matrix. Additionally, the increased diffusivity improves the mass transfer of dissolved compounds, thus improving the extraction efficiency.
3. Examples of plant derived food polysaccharides that are currently used as food ingredients
Many plant-based polysaccharides play a key role in the formation of desired food structures in various products, as they are authorized for food use as added ingredients or additives. The following paragraphs briefly present three polysaccharides that have similarities, either structural- or origin-based, to spruce GGM. Arabinogalactan from larch is a hot water extracted wood polysaccharide that has a relatively low molar mass. Konjac glucomannan and guar galactomannan have monosaccharide compositions similar to those of GGM; they are widely used in the food industry as a thickening and structuring agent.3.1 Arabinogalactan
Arabinogalactan, a hemicellulose from western larch (Larix occidentalis), is used as a food hydrocolloid in Northern America, Japan and Australia. Larch arabinogalactan has a relatively low molar mass (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), it is highly water-soluble and solutions of high concentration (up to 50%) can be produced with very low viscosities.25,26 For the extraction process of arabinogalactan, wood is debarked, chipped and then extracted with hot water in a two-stage extraction process equipped with screw presses. The extraction process yields an aqueous product, having a concentration of about 50% of arabinogalactan. Products containing arabinogalactan can be used in a liquid form (preserved with potassium sorbate) or in a spray dried powder form, after undergoing hydrogen peroxide and potassium hydroxide purification and decolorization.25 Arabinogalactan from larch was accepted as a food additive for use in food as an emulsifier, a stabilizer, and a binding or viscosity agent for essential-oils, flavours, non-nutritive sweeteners, salad dressings and pudding mixes, in the USA, in 1965. In addition, arabinogalactan has a GRAS (generally regarded as safe) status. However, it has not been used as a food additive in the EU. The powder form (decolorized) food-grade arabinogalactan contains 4.74% moisture, 89.3% carbohydrates, 5.27% ash, 0.47% protein and 0.23% fat. Arabinogalactan can be naturally found in common foods, such as coffee beans, soybean seeds, rapeseed, apple, wheat flour and rye flour.
000 g mol−1), it is highly water-soluble and solutions of high concentration (up to 50%) can be produced with very low viscosities.25,26 For the extraction process of arabinogalactan, wood is debarked, chipped and then extracted with hot water in a two-stage extraction process equipped with screw presses. The extraction process yields an aqueous product, having a concentration of about 50% of arabinogalactan. Products containing arabinogalactan can be used in a liquid form (preserved with potassium sorbate) or in a spray dried powder form, after undergoing hydrogen peroxide and potassium hydroxide purification and decolorization.25 Arabinogalactan from larch was accepted as a food additive for use in food as an emulsifier, a stabilizer, and a binding or viscosity agent for essential-oils, flavours, non-nutritive sweeteners, salad dressings and pudding mixes, in the USA, in 1965. In addition, arabinogalactan has a GRAS (generally regarded as safe) status. However, it has not been used as a food additive in the EU. The powder form (decolorized) food-grade arabinogalactan contains 4.74% moisture, 89.3% carbohydrates, 5.27% ash, 0.47% protein and 0.23% fat. Arabinogalactan can be naturally found in common foods, such as coffee beans, soybean seeds, rapeseed, apple, wheat flour and rye flour.
3.2. Konjac glucomannan
Konjac glucomannan (E425) is a high molar mass (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2000
000–2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) polysaccharide, which is extracted from the roots of Amorphophallus konjac. Due to the high molar mass, konjac glucomannan is an ideal thickener and emulsion stabilizer (enhances the viscosity of the continuous phase in oil-in-water emulsions). Konjac glucomannan consists of (1→4)-linked linear chains containing both β-D-mannopyranosyl and β-D-glucopyranosyl units in a ratio of 1.6
000 g mol−1) polysaccharide, which is extracted from the roots of Amorphophallus konjac. Due to the high molar mass, konjac glucomannan is an ideal thickener and emulsion stabilizer (enhances the viscosity of the continuous phase in oil-in-water emulsions). Konjac glucomannan consists of (1→4)-linked linear chains containing both β-D-mannopyranosyl and β-D-glucopyranosyl units in a ratio of 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A low amount of branching (8%) occurs through β-(1→6)-glucosyl linkages. Acetyl groups attached to chains increase the water solubility of the polymer. Konjac glucomannan is widely used in food emulsions and as food thickeners as well as food supplements.27 Konjac glucomannan has been shown to contribute to the reduction of body weight in the context of an energy-restricted diet (health claim approved by EC).28 A synergistic polysaccharide complex containing konjac glucomannan, alginate and xanthan has higher viscosity in hydrated form than the individual polysaccharides and is marketed as a source of dietary fibre that can be used in various foods and beverages as well as in dietary supplements.29
1. A low amount of branching (8%) occurs through β-(1→6)-glucosyl linkages. Acetyl groups attached to chains increase the water solubility of the polymer. Konjac glucomannan is widely used in food emulsions and as food thickeners as well as food supplements.27 Konjac glucomannan has been shown to contribute to the reduction of body weight in the context of an energy-restricted diet (health claim approved by EC).28 A synergistic polysaccharide complex containing konjac glucomannan, alginate and xanthan has higher viscosity in hydrated form than the individual polysaccharides and is marketed as a source of dietary fibre that can be used in various foods and beverages as well as in dietary supplements.29
3.3 Guar gum
Guar gum (E412) is the ground endosperm of guar (Cyamopsis tetragonolobus) beans. Guar beans grow mainly in India, Pakistan, the USA, Australia and African countries. The main component in guar gum is galactomannan, which consists of a (1→4)-linked β-D-mannopyranosyl backbone carrying α-D-galactopyranol substituents at the position of O-6. The mannose-to-galactose ratio in guar galactomannan is 1.6. Structurally similar galactomannans (with different mannose-to-galactose ratios) can be found in other plants, such as the Ceratonia siliqua (locust bean gum), Caesalpinia spinose (tara gum) and Trigonella foenum graecum (fenugreek gum). Guar gum is used extensively in the food industry as thickening and stabilizing agents. Food-grade guar gum powder contains ≥75% of galactomannan. Additionally, guar gum contains crude protein (5–6%), moisture (8–15%), crude fibre (2.5%), ash (0.5–0.8%) and minor amounts of lipids.21 Due to the high molar mass of guar gum, low amounts, <1% of the food weight, are commonly used.30 Foods that provide a daily intake of 10 g of guar gum are permitted to health claim that “Guar gum contributes to the maintenance of normal blood cholesterol levels”.31In conclusion, all three polysaccharides, which have similarities to the spruce GGM, are accepted for food use. Arabinogalactan is wood-based, and the extraction process is similar to PHWE used to extract GGM; additionally, its molar mass is in the same range as the molar mass for GGM. Konjac glucomannan and guar gum are both mannans, and thus, are structurally similar to GGM. Based on these examples, we believe that GGM has potential in the food industry, as a multi-functional food ingredient in the future. Before GGM can be used as a food ingredient, however, its safety needs to be evaluated, as requested by the EU Novel Food regulation.
4. Legislation regarding Novel Food ingredients in Europe
4.1 Application for authorization of a Novel Food
All new materials intended for food purposes need to meet the safety requirements regulated by the EU legislation. According to the European Commission, Novel Food is defined as: “food that has not been consumed to a significant degree by humans in the EU prior to 1997, when the first Regulation on Novel Food came into force. ‘Novel Food’ can be newly developed, innovative food or food produced using new technologies and production processes as well as food traditionally eaten outside of the EU”.32 In Europe, the European Parliament and the Council have laid down the updated regulation concerning Novel Foods and Novel Food ingredients (regulation (EC) no. 2015/2283).33 According to the regulation (article 7), Novel Foods and Novel Food ingredients must not present a danger for the consumer, mislead the consumer or differ from the foods or food ingredients that they are intended to replace to such an extent that their normal consumption would be nutritionally disadvantageous for the consumer. Before the product that is regarded as a Novel Food/Novel Food ingredient can be released into the market, an application needs to be submitted to the European Commission. The EFSA has the responsibility of evaluating the safety of the Novel Food and forwarding the opinion to the Commission.34 The Commission's decision defines the scope of the authorization and establishes the conditions of use for the food or food ingredient, the designation of the food or food ingredient and its specification and specific labelling requirements. Consumers need to be informed of any characteristic or food properties, such as composition, nutritional value or nutritional effects and intended uses of the food.4.2 Safety evaluation of Novel Foods
Obviously, Novel Foods must be safe for consumers. If the authorization of a Novel Food is applied for a certain ingredient, the data needed to carry out the safety assessment have to be provided in the application as directed by the EFSA.34 The list of required information and data is presented in Table 1. All of the analyses and tests should be performed in a facility that can certify the data, and validated methods (e.g., Association of Analytical Communities, AOAC) should be used whenever possible. The compositional data should be based on the analyses from at least five different batches of the product. Considerations regarding the safety evaluation of GGM are presented in the following section.| List of required information/data | Further details/remarks |
|---|---|
| a Depends on the nature of the Novel Food (chemical substance, isolate, polymer, mixture, etc.). b Minimun requirements include data on absorption, genotoxicity (in vitro) and toxicity (an extended 90-day toxicity study on rats). c Includes data on subchronic and chronic toxicity, carcinogenicity, and reproductive and developmental toxicity. | |
| Generic description of the Novel Fooda | e.g., chemical name, structure, CAS number, synonyms, molecular formula, molar mass, particle size, shape and distribution |
| Detailed description on production process | Special focus on potential by-products, impurities or contaminants that could cause safety concerns |
| Detailed qualitative and quantitative data on the composition | Unidentified constituents should be as low as possible |
| Stability evaluation during storage | For food ingredients the stability in the processed food should be investigated |
| Specifications defining key parameters (and limits) which characterize the identity of the Novel Food | |
| Limits acceptable for impurities and degradation products | |
| Description on the history of use of the Novel Food | Studies on similar foods from the same or the related sources should be included |
| Estimation for intakes | Intakes from different sources (natural, added) should be taken into account |
| Estimation to exposure to undesirable substances | |
| Data on toxicokinetic testing (adsorption, distribution, metabolism, and excretion, ADME)b | |
| Nutritional information | |
| Toxicological informationc | |
| Description of possible allergens for protein-containing products | |
5. Safety considerations for GGM
5.1 Composition of spruce GGM extracted using pressurized hot water extraction
GGM extracted using the PHWE procedure is highly potential for food products because the extraction process utilizes only water as the extraction solvent. The spruce sawdust contains 42% cellulose, 23% hemicelluloses, 26% lignin, 3% extractives and 6% other compounds, mainly acetyl groups from hemicelluloses and small amounts of proteins and ash.22 Lignin is a complex three-dimensional macromolecule (“polyphenolic compound”) which consists of polymerized monolignols: p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol. The dominating lignin monomer in softwood lignin is coniferyl alcohol.36 Extractives are non-structural components in wood and their content is higher in bark, leaves and roots than in stem wood. Extractives contain both aliphatic and alicyclic compounds as well as phenolic compounds. The former includes terpenes and terpenoids, esters of fatty acids, fatty acids, alcohols and alkanes. The latter category includes simple phenols, stilbenes, lignans, isoflavones, condensed tannins, flavonoids and hydrolyzable tannins.37 The composition of spruce heartwood extractives (petroleum ether extracted) is presented in Table 2. The hemicellulose fraction is enriched in spruce GGM extracted by PHWE (Fig. 4a). The extract contains 75% hemicelluloses, 16.7% lignin, 6.7% extractives and 1.7% other compounds (such as proteins, acetic acid and ash). It should be noted that the composition of the laboratory scale and the pilot scale extracted material is very similar. The monosaccharide compositions for both the spruce sawdust and extract (from pilot and laboratory scale extractions) are presented in Fig. 4b. Both the sawdust and extract contain mainly mannose, glucose, galactose and xylose. In the extract, mannose, galactose and glucose originate from galactoglucomannans. Additionally, spruce hemicellulose contains arabinoglucuronoxylan,3 which consists of a (1→4)-linked β-D-xylopyranosyl backbone with (1→2)-linked 4-O-methyl-α-D-glucopyranosyl uronic acid and α-L-arabinofuranose linked to C-3 of xylose residues as side groups. No detailed data on the composition of “other compounds” exist.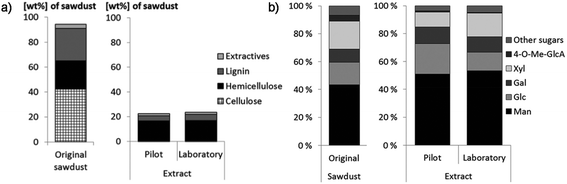 | ||
| Fig. 4 (a) The chemical composition and (b) monosaccharide composition of spruce sawdust and PHWE GGM. Other sugars include arabinose, rhamnose, glucuronic acid and galacturonic acid. The pilot scale extraction was developed after successful small-scale experiments in the laboratory. Reprinted from ref. 22, courtesy of the Royal Society of Chemistry. | ||
| Component | mg per g of wood |
|---|---|
| Fatty acids | 3.29 |
| Resin acids | 0.95 |
| Sterols | 0.94 |
| Triterpene alcohols | 0.13 |
| Diterpene alcohols and aldehydes | 0.29 |
| Alkyl ferulates | 0.19 |
| Glyceryl residues | 0.22 |
| Total | 6.0 (0.6%) |
Based on the studies of extracts that have structural similarities to spruce GGM, most of the compounds present in the GGM extract are probably safe for humans and animals. One example is the wood-based flavonoid taxifolin, which is extracted from Dahurian larch (Larix gmelinii) and has been recently evaluated by the EFSA and approved for food use.38 However, low amounts of some undesirable substances may also be present in the extract. Prior to possible food use of GGM, the levels of heavy metals (lead, arsenic, cadmium and mercury) in the extract should be analysed. The limits for these heavy metals in plant-based food range from 0.1 to 0.3 mg per kg wet weight.39,40 In addition, microbial safety should be ensured. Because no solvents are involved in the PHWE process, no solvent residues are expected to be present in the GGM extract. In addition to heavy metals and possible microbial contaminants, furfural and furfural diethylacetal, which are formed from polysaccharides containing pentose or hexose sugars during acid hydrolysis and heating, are undesirable substances that may be present in the extract. Because high temperatures are used in the PHWE process, small amounts of furfurals may form during the extraction of spruce GGM. In the extract of birch, obtained by the PHWE process, 79 μg l−1 of furfurals were detected when the extraction temperature was 190 °C.41 Also, resin acids, especially abietic acid, levopimaric acid, palustric acid, neoabietic acid, dehydroabietic acid, pimaric acid, isopimaric acid and sandaracopimaric acid, which are included in the wood extractives (Table 2), may be toxic,42 but their total amount in the extracted GGM is likely to be very small.
5.2 Molar mass
The molar mass of PHWE GGM (ethanol precipitated) is in the range of ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.11 Wood hemicelluloses are known to have a lower molar mass than, e.g., the hemicelluloses from monocotyledonous plants, such as cereals.44,45 However, the PHWE process may have caused some degradation of the GGM chains because GGM from spruce thermomechanical pulping process water has a higher molar mass, in the range of 20
000 g mol−1.11 Wood hemicelluloses are known to have a lower molar mass than, e.g., the hemicelluloses from monocotyledonous plants, such as cereals.44,45 However, the PHWE process may have caused some degradation of the GGM chains because GGM from spruce thermomechanical pulping process water has a higher molar mass, in the range of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–40
000–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. Another explanation for the discrepancy between the molar masses could be that the PHWE process extracts lower molar mass polymers than what can be recovered from thermomechanical pulping process water.9,12,46 Determining the molar mass of GGM is not a straightforward task because GGM forms colloidal aqueous solutions already in relatively low concentrations of ≥0.1%.46 Molar masses (or molar mass distributions) are most often determined using a liquid chromatographic method, namely size-exclusion chromatography, which requires the samples to be soluble (in the form of single chains) in the mobile phase. Thus, due to the colloidal nature of the aqueous GGM solutions, other solvents, such as dimethyl sulfoxide, are commonly used in size-exclusion chromatographic analyses of GGM.47
000 g mol−1. Another explanation for the discrepancy between the molar masses could be that the PHWE process extracts lower molar mass polymers than what can be recovered from thermomechanical pulping process water.9,12,46 Determining the molar mass of GGM is not a straightforward task because GGM forms colloidal aqueous solutions already in relatively low concentrations of ≥0.1%.46 Molar masses (or molar mass distributions) are most often determined using a liquid chromatographic method, namely size-exclusion chromatography, which requires the samples to be soluble (in the form of single chains) in the mobile phase. Thus, due to the colloidal nature of the aqueous GGM solutions, other solvents, such as dimethyl sulfoxide, are commonly used in size-exclusion chromatographic analyses of GGM.47
5.3 Physical properties
Rheological properties of aqueous solutions of GGM obtained with different extraction procedures have been studied.11,46,48 Due to the low molar mass of PHWE GGM (please see the previous paragraph), the viscosity is also low and exhibits Newtonian behaviour, even for GGM contents of up to 30 wt%.11 The low viscosity of GGM is also seen in Fig. 5a, which shows the specific viscosity of the aqueous GGM solutions as a function of the coil overlap parameter c[η] (which describes the space that molecules occupy in the solution), where the intrinsic viscosity [η] was 16 ml g−1. At low concentrations, below the coil overlap parameter ∼2, the viscosity increases moderately, which is expected for dilute polysaccharide solutions. At the coil overlap concentration and above that, interactions between the polysaccharide chains exist and the viscosity increases faster as a function of the concentration compared to the dilute solutions. One way to estimate the overlap concentration is to take the inverse of the intrinsic viscosity. Thus, the overlap concentration of PHWE GGM is ∼65 g l−1. This concentration is significantly higher than the concentrations of polysaccharides that are commonly added to food products. Due to the low viscosity of the GGM solutions, the viscosity will most likely not be a safety concern regarding the food use of GGM. However, there is a past instance where konjac glucomannan-containing jelly candies were withdrawn from the market because they caused a potential choking hazard to consumers, particularly to the children and elderly.49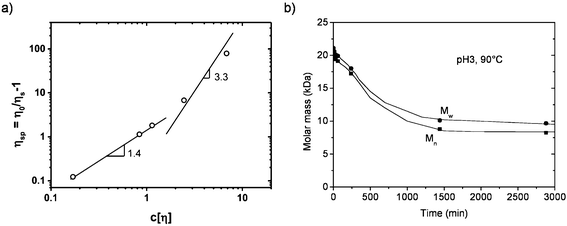 | ||
| Fig. 5 (a) The specific viscosity of aqueous PHWE galactoglucomannan (GGM) solutions as a function of the coil overlap parameter c[η]. The intrinsic viscosity [η] was 16 ml g−1. Reprinted from ref. 11, courtesy of the Royal Society of Chemistry. (b) The effect of pH on the molar mass (Mw, weight-average molar mass; Mn, number-average molar mass) of GGM. Reprinted with permission from ref. 50. Copyright (2008) American Chemical Society. | ||
5.4 Stability during storage
Food processing conditions are often acidic and include treatments at high temperatures. Such treatments may cause the degradation of polysaccharides, and thus, stability studies have been conducted to see how different polysaccharides behave under acidic conditions and/or at high temperatures. Xu et al. investigated the stability of GGM under acidic conditions of pH 1, 2 and 3 at 90 °C.50 The degradation of GGM occurred at all the pH values tested. At the lowest pH of 1, polymeric GGM was depolymerized to monomers in two days. The degradation at the higher pH values of 2 and 3 was less drastic; at pH 3, the molar mass was reduced by a factor of two after one day and no further reduction in the molar mass was observed (Fig. 5b). In addition, the effect of temperature on the degradation of GGM was studied at pH 1. Compared to the changes of the molar mass at 90 °C, the reduction of the molar mass was significantly lower at temperatures of 70, 50, and 37 °C. At 50 °C, the molar mass was halved after two days, after which no reduction was observed. No degradation occurred at 37 °C or lower temperatures. The pH of aqueous GGM solutions has been reported to be around 5.46 The pH of many food matrices is, however, lower, and thus, stability tests of GGM in food matrices should be conducted before releasing it to the market.The stability of another mannan, guar gum, has also been studied. Guar gum was found to be relatively stable in the acidic environment, even at high temperatures. Rao et al.51 noticed that a long heat treatment at a temperature range from around 100 °C to 130 °C caused the mild depolymerization of guar gum. The hydrolysis of the polymer caused a decrease in viscosity and hydrodynamic volume, which is described by the intrinsic viscosity. When the 1% (w/v) guar gum solution was heat treated at 121 °C for 10 min, the intrinsic viscosity decreased almost one third. In addition, the amount of reducing sugars increased during the heat treatment. Cheng et al.52 followed the degradation of guar gum under acidic conditions at pH 1 for 2, 4, 10.5, and 15 h. After the 15 h treatment, the intrinsic viscosity of guar gum decreased almost sixfold. It should be noted that in these two stability studies only a mild degradation took place without any changes in the chemical structure of guar gum.
5.5 Proposed uses and anticipated intake
GGM has proved to be an efficient multi-functional emulsifier and stabilizer and could thus be used in various food products (both oil-in-water and water-in-oil types of emulsions). GGM could be used as an alternative to guar gum or gum arabic, which are commonly used in the food industry for emulsifying and emulsion stabilizing purposes. The optimal GGM-to-oil ratio depends on the oil content of the product. However, a GGM-to-oil ratio of around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 is sufficient for products containing a relatively high oil content, such as salad dressings (in which the oil content could be approximately 40%) as well as products with a lower oil content (e.g., drink products with an oil content of <5%). These GGM-to-oil ratios have been proved to work in test emulsions9–11 and give indication on the ratios that would also be applicable in “real” food emulsions.
5 is sufficient for products containing a relatively high oil content, such as salad dressings (in which the oil content could be approximately 40%) as well as products with a lower oil content (e.g., drink products with an oil content of <5%). These GGM-to-oil ratios have been proved to work in test emulsions9–11 and give indication on the ratios that would also be applicable in “real” food emulsions.
Typical food emulsions include dressings, sauces, margarine, fermented milk products and drinks. The estimated use levels of GGM in different products, based on the GGM-to-oil ratios discussed in the previous paragraph, could range from 1% to 10%, depending on the oil content. Based on the average food consumption of the Finnish population (National FINDIET survey, 2012; The EFSA Comprehensive European Food Consumption Database), the estimated daily intake of GGM would be 32 mg per kg bw per day, assuming that GGM would replace all other polysaccharide-based emulsifiers in the foods that belong to the categories of dressings, margarines, fermented milk products and milk product imitates. Assuming a 70 kg body weight, the average daily intake would be 2.2 g per person per day. This value is a reasonable estimate; in the UK, the mean exposure for depolymerized guar gum, which has functional similarities to GGM, was estimated to be approximately 2.9 g per person per day, and globally, the exposure was estimated to range between 41 and 57 mg per kg bw per day.53 In addition to its use as an ingredient in food products, GGM could also be used as a food supplement. For example, a similar product is (hot water extracted) larch arabinogalactan, which is used as a source of dietary fibre in food products and food supplements (mainly in the USA).
5.6 Toxicological information
To date, there is no toxicological information on GGM. When assessing the nutritional and toxicological impact of a potential Novel Food, data on absorption, distribution, metabolism and excretion (ADME) are needed.34 As a plant hemicellulose, GGM is included in the definition of dietary fibre, which is not digested or absorbed in the small intestine but may be partly fermented in the large intestine by the microbes. Polyphenolic lignin, which is present in the GGM extract in addition to the hemicellulose fraction, is also included in dietary fibre. Although the toxicity of GGM has not been studied, supporting evidence for non-toxicity can be drawn from the feeding studies in which PHWE GGM was fed to sheep.54 In these in vivo studies, it was noted that GGM was highly fermentable and caused no harmful effects to the ruminants. In addition, plenty of data can be found from the literature for structurally related mannans, namely for konjac glucomannan and for guar galactomannan. Both short-term toxicity and long-term toxicity tests have been conducted for konjac glucomannan. Based on the results of these toxicological studies, the NOAEL (no-observed adverse effect level; “the highest experimental point that is without adverse effect”55) value for konjac glucomannan has been set to 500 mg per kg bw per day. Based on the 4-hour cytotoxicity assay, konjac glucomannan is not considered to be cytotoxic.56Toxicological data exist for several galactomannans that are accepted as food constituents (guar gum, locust bean gum and tara gum). These galactomannans were not carcinogenic to mice or rats. The carcinogenicity testing of guar galactomannans included a 103-week carcinogenicity study in rats and mice. A dosage of 1250 or 2500 mg per kg bw per day for rats and 3600 or 7200 mg per kg bw per day for mice was administered for 103 weeks. These amounts did not induce cancer.57,58 Similarly, a 2-year feeding study was conducted for locust bean gum and tara gum, and, in these studies, no carcinogenic effects were detected.58 The highest tested dosages were based on the NOAEL values.
In addition to the murine studies, a human clinical study was conducted in which a daily dosage of 30 g of guar gum was administered to non-insulin dependent diabetic patients for 16 weeks.59 This study showed that there were no changes in the hematologic, hepatic, or renal functions. Furthermore, no change in the lipid, protein or mineral metabolism, or electrolyte balance was observed. Thus, the results of this clinical study suggested that guar gum can be consumed in relatively large quantities without adverse effects.
The toxicity of two oxidized depolymerized guar gums prepared by alkaline hydrolysis was tested using a 90-day toxicity study in which guar gums were given to weanling rats using dosages of 0 (control), 1000, and 2500 mg per kg bw per day.53 No adverse effects to partially depolymerized guar gum were found when growth, food consumption and chemical, clinical and histopathological examinations of the animals were conducted.
Arabinogalactan is a wood-based (extracted from larch) polysaccharide that has been used as a food ingredient. In an acute toxicity study, the LD50 value (“lethal dose 50%”) of 12.9 mg kg−1 was established. A 30-day toxicity study indicated that arabinogalactan was innocuous to rats when fed to rats for 30 days at dosages of up to 25%. A six-month chronic toxicity study of arabinogalactan was conducted in beagle dogs. The study showed that there were no significant gross or histological alterations in the group that received arabinogalactan.25
Furfurals occur naturally in many food items, such as fruit, tea, coffee and cocoa, and may also be present in the GGM extract in minor amounts. In addition to its natural occurrence, furfurals are also used as a flavouring substance in many food categories. The EFSA has established an ADI (acceptable daily intake) value for furfural and furfural diethylacetal (the latter is rapidly converted into furfural at the physiological pH) of 0.5 mg per kg bw. This value is based on applying a safety factor of 100 to the NOAEL value of 54 mg per kg bw per day, which was obtained from a 90-day hepatotoxicology study in rats.60 Many short-term and long-term toxicity studies have been conducted for furfurals. The oral LD50 for furfural has been reported to be 127 mg per kg bw in rats61 and 333 mg per kg bw in mice.62In vivo toxicity studies have provided evidence showing that furfural did not induce genotoxicity in male mice. However, tumours were observed in male mice in long-term carcinogenicity studies, causing a concern of chronic hepatotoxicity. Hepatocellular tumours were not observed in a long-term rat study, but rats were more sensitive to liver toxicity.60 Due to the safety concerns related to furfurals, their levels in GGM extracts should be monitored; however, the exposure, based on the estimated intakes of GGM, will likely remain well below the ADI value of 0.5 mg per kg bw.
Spruce heartwood contains 0.95 mg g−1 of wood (0.095%) resin acids (Table 2).43 Resin acids may adversely affect humans. Abietic acid has been responsible for asthmatic reactions. Additionally, exposure to resin acids can cause chronic lung damage.63 However, resin acids containing additive E445, a glycerol ester of wood rosin, has been accepted for food use (emulsifier/density adjustment agent). The glycerol ester of wood rosin is a complex mixture of glycerol di- and tri-esters of resin acids from wood rosin with a residual fraction of glycerol monoesters. In addition to esters, E445 contains residual free resin acids. Based on the toxicological studies, an ADI value of 12.5 mg per kg bw per day has been established. In beverages, the maximum acceptable level of E445 is 100 mg l−1.64 The amount of extractives in PHWE GGM is low (Fig. 4a), and thus, it is very unlikely that resin acid would cause a safety concern if GGM were to be accepted for food use.
5.7 Allergenicity
Food-grade polysaccharide extracts often contain some amounts of residual proteins. A good example of such an extract is guar gum, which contains proteins (less than 10%).65 Protein-containing food ingredients may cause allergic reactions. A few reports indicate that exposure to guar gum or guar gum dust has caused allergic reactions and occupational asthma.66,67 Due to a limited number of reports indicating guar gum as a source of allergen, guar gum cannot be considered as a significant allergen in food. Similarly, it has been reported that softwood dust may cause occupational allergies, but the food-grade taxifolin extract from larch wood is not considered an allergen.38,68 The protein content of GGM has been reported to be ≤1% (wt%).69 Based on the proposed use level of GGM, the protein exposure from the GGM extract would be very low, ≤22 mg per person per day.6. Potential health-promoting effects of GGM
GGM may have several potential health-promoting effects, including functioning as prebiotic, antioxidant, and anti-inflammatory agents. The GGM extract consists mainly of polysaccharides. Polysaccharides are included in dietary fibre with other edible, non-digestible parts of plants, such as oligosaccharides and lignin. Dietary fibre is known to promote beneficial physiological effects, including laxation, and/or blood cholesterol attenuation and/or blood glucose attenuation.2 According to the health claims,70 claims on individual fibre constituents and health effects have been authorized. For example, these include konjac glucomannan (contributing to the maintenance of normal blood cholesterol levels as well as to weight loss in the context of an energy restricted diet), guar gum (contributing to the maintenance of normal blood cholesterol levels), wheat endosperm arabinoxylan (consumption as part of a meal contributes to a reduction of the blood glucose rise after that meal), and beta-glucans (contributing to the maintenance of normal blood cholesterol levels).70 Spruce-derived carbohydrates may have prebiotic functions in the large intestine.71 It has already been demonstrated that Bifidobacterium and Lactobacillus species are able to ferment hemicellulose-derived saccharides in vitro, and that GGM possesses prebiotic functions.72 Significant stimulatory effects on the growth rate and concentration of common probiotics, such as Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus rhamnosus, were observed when galactoglucomannans were in growth media.As previously mentioned, wood contains polyphenolic lignin and a low amount of extractives, including some phenolic compounds. Phenolic compounds are also found in (edible) plants, such as in cereals (the total phenolic content in common cereals ranges from ∼15 mg g−1 to ∼20 mg g−1 (ref. 73)). Immunomodulating and radical-scavenging activities of GGM extracts containing phenolic compounds have been investigated in vitro. Both acetylated and non-acetylated spruce GGM had dose-dependent biological responses in the lymphocyte transformation test (GGM enhances the proliferation of rat thymocytes). Acetylated GGM also possessed radical-scavenging activity. The antiradical activity of polysaccharide extracts has been postulated to correlate with the total phenolic content of the extract.4 Phenolic wood extractives, such as flavonoids and lignans, are also powerful antioxidants.74 However, the results on the relation between antioxidant activity and the phenolic content of various plant extracts are not consistent.4 Antioxidant activity has also been reported for polysaccharides (β-glucan type) without any phenolics as coextracts.75,76In vivo studies on broiler chicks77,78 have shown that extracts containing GGM oligosaccharides improved the intestinal immune response towards infections caused by common pathogens. Oligosaccharides are present in the GGM fractions that are not ethanol precipitated, and thus, these “structurally heterogeneous” GGM fractions may have a more significant bioactive role in comparison with the purified GGM fractions. Additionally, other galactose containing glucomannans have shown immunomodulating effects. Glucomannan from the mushroom Cordyceps militaris has been shown to activate the macrophages that recognize and kill tumour cells.79,80
PHWE spruce GGM was recently patented for medicinal purposes.5 The GGM extract is considered to act as a therapeutic agent for preventing and treating the symptoms of lower urinary track disease.6 Based on the studies conducted on rats, the GGM extract has shown to reduce prostate cancer cell proliferation in a concentration-dependent manner, decrease the cancer area measured from the histological sections (prostate cancer), improve the signs of obstructive voiding (decrease basal bladder pressure, increase urinary flow rates and increase voided volume) and reduce the abdominal (pelvic) pain by reducing the feeling of pain, i.e., increasing the pain threshold. It is not yet clear which compounds or mixture of compounds in the extract are behind the bioactive effects found in the animal experiments. However, it was postulated that a bladder–gut interaction exists, which means that the neural link between pelvic organs modulates their physiological function.5,6 Many polysaccharides, such as GGM, are fermented in the colon to, e.g., short-chain fatty acids, which are known to promote gut health.
As described in the previous paragraphs, in vitro and in vivo studies indicate that GGM may act as a health-promoting compound. It should be noted, however, that, to date, no human clinical studies on the health-effects of GGM have been conducted.
7. Conclusions
To evaluate the safety of Novel Food ingredients, their origin, isolation method, composition, purity, stability, physicochemical properties, toxicology and allergenicity must be considered. Spruce GGM is an example of a non-conventional but potential ingredient for food applications. The multi-functionality of GGM suggests that it could replace other, less effective food emulsion stabilizers. Polysaccharides that have similarities, either structural- or origin-based, to spruce GGM are already abundantly used in processed food. The safety evaluations of these plant-based materials can be considered as guidelines for the safety evaluations of GGM. The PHWE method for GGM extraction is simple and safe, since only water is used as a solvent and chemicals are avoided. Feeding studies have provided evidence that GGM, a phenolic-rich polysaccharide, is not harmful to animals. Future research is needed to evaluate the toxicity of GGM, as requested by the EU legislation on food safety, before GGM can be approved as a food ingredient. Spruce GGM, an abundant renewable bioresource, is envisioned as a future food ingredient that may pave the way for the safety evaluation and commercialisation of other potential polysaccharide-based plant extracts.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We would like to thank Mari Lehtonen for fruitful discussions during the writing process. The Academy of Finland (project no. 305517) is acknowledged for funding.References
- A. M. Stephen, G. O. Phillips and P. A. Williams, Food Polysaccharides and Their Applications, CRC Press, Boca Raton, 2006 Search PubMed.
- AACC, The definition of dietary fibre, Cereal Foods World, 2001, 46, 112–126 Search PubMed.
- E. Sjöström, Wood Chemistry Fundamentals and Applications, Academic Press, Inc., San Diego, 1993 Search PubMed.
- A. Ebringerová, Z. Hromádková, V. Hříbalová, C. Xu, B. Holmbom, A. Sundberg and S. Willför, Norway spruce galactoglucomannans exhibiting immunomodulating and radical-scavenging activities, Int. J. Biol. Macromol., 2008, 42, 1–5 CrossRef PubMed.
- Montisera Ltd, Fin. Pat, FI20126175, 2015 Search PubMed.
- Y. Konkol, H. Vuorikoski, J. Tuomela, B. Holmbom and J. Bernoulli, Galactoglucomannan-rich hemicellulose extract from Norway spruce (Picea abies) exerts beneficial effects on chronic prostatic inflammation and lower urinary tract symptoms in vivo, Int. J. Biol. Macromol., 2017, 101, 222–229 CrossRef PubMed.
- S. Willför, K. Sundberg, M. Tenkanen and B. Holmbom, Carbohydr. Polym., 2008, 72, 197–210 CrossRef.
- S. Cui, Food Carbohydrates: Chemistry, Physical Properties, and Applications, CRC Press, Boca Raton, 2005 Search PubMed.
- K. S. Mikkonen, C. Xu, C. Berton-Carabin and K. Schroën, Spruce galactoglucomannans in rapeseed oil-in-water emulsions: Efficient stabilization performance and structural partitioning, Food Hydrocolloids, 2016, 52, 615–624 CrossRef CAS.
- M. Lehtonen, S. Teräslahti, C. Xu, M. P. Yadav, A.-M. Lampi and K. S. Mikkonen, Spruce galactoglucomannans inhibit lipid oxidation in rapeseed oil-in-water emulsions, Food Hydrocolloids, 2016, 58, 255–266 CrossRef CAS.
- K. S. Mikkonen, D. Merger, P. Kilpeläinen, L. Murtomäki, U. S. Schmidt and M. Wilhelm, Determination of physical emulsion stabilization mechanisms of wood hemicelluloses via rheological and interfacial characterization, Soft Matter, 2016, 12, 8690–8700 RSC.
- K. S. Mikkonen, M. I. Heikkilä, H. Helen, L. Hyvönen and M. Tenkanen, Spruce galactoglucomannan films show promising barrier properties, Carbohydr. Polym., 2010, 79, 1107–1112 CrossRef CAS.
- K. S. Mikkonen, J. S. Stevanic, C. Joly, P. Dole, K. Pirkkalainen, R. Serimaa, L. Salmen and M. Tenkanen, Composite films from spruce galactoglucomannans with microfibrillated spruce wood cellulose, Cellulose, 2011, 18, 713–726 CrossRef CAS.
- K. S. Mikkonen, M. I. Heikkilä, S. M. Willför and M. Tenkanen, Films from glyoxal-crosslinked spruce galactoglucomannans plasticized with sorbitol, Int. J. Polym. Sci., 2012, 482810, 8 Search PubMed.
- K. S. Mikkonen, J. Schmidt, A. Vesterinen and M. Tenkanen, Crosslinking with ammonium zirconium carbonate improves the formation and properties of spruce galactoglucomannan films, J. Mater. Sci., 2013, 48, 4205–4213 CrossRef CAS.
- V. Kisonen, K. Prakobna, C. Xu, A. Salminen, K. S. Mikkonen, D. Valtakari, P. Eklund, J. Seppälä, M. Tenkanen and S. Willför, Composite films of nanofibrillated cellulose and O-acetyl galactoglucomannan (GGM) coated with succinic esters of GGM showing potential as barrier material in food packaging, J. Mater. Sci., 2015, 50, 3189–3199 CrossRef CAS.
- W. Zhao, K. Odelius, U. Edlund, C. Zhao and A. Albertsson, In situ synthesis of magnetic field-responsive hemicellulose hydrogels for drug delivery, Biomacromolecules, 2015, 16, 2522–2528 CrossRef CAS PubMed.
- L. Maleki, U. Edlund and A. Albertsson, Green semi-IPN hydrogels by direct utilization of crude wood hydrolysates, ACS Sustainable Chem. Eng., 2016, 4, 4370–4377 CrossRef CAS.
- S. Alakalhunmaa, K. Parikka, P. A. Penttilä, M. T. Cuberes, S. Willför, L. Salmen and K. S. Mikkonen, Softwood-based sponge gels, Cellulose, 2016, 23, 3221–3238 CrossRef CAS.
- N. Rosa-Sibakov, T. K. Hakala, N. Sözer, E. Nordlund, K. Poutanen and A. Aura, Birch pulp xylan works as a food hydrocolloid in acid milk gels and is fermented slowly in vitro, Carbohydr. Polym., 2016, 154, 305–312 CrossRef CAS PubMed.
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), A. Mortensen, F. Aguilar, R. Crebelli, A. Di Domenico, M. J. Frutos, P. Galtier, D. Gott, U. Gundert-Remy, C. Lambré, J. Leblanc, O. Lindtner, P. Moldeus, P. Mosesso, A. Oskarsson, D. Parent-Massin, I. Stankovic, I. Waalkens-Berendsen, R. A. Woutersen, M. Wright, M. Younes, L. Brimer, P. Peters, J. Wiesner, A. Christodoulidou, F. Lodi, A. Tard and B. Dusemund, Re-evaluation of guar gum (E 412) as a food additive, EFSA J., 2017, 15, 4669 Search PubMed.
- P. O. Kilpeläinen, S. S. Hautala, O. O. Byman, L. J. Tanner, R. I. Korpinen, M. K.-J. Lillandt, A. V. Pranovich, V. H. Kitunen, S. M. Willför and H. S. Ilvesniemi, Pressurized hot water flow-through extraction system scale up from the laboratory to the pilot scale, Green Chem., 2014, 16, 3186–3194 RSC.
- S. Von Schoultz, Fin, Pat, FI2013/050723, 2013 Search PubMed.
- S. Willför, P. Rehn, A. Sundberg, K. Sundberg and B. Holmbom, Recovery of water-soluble acetyl-galactoglucomannans from mechanical pulp of spruce, Tappi J., 2003, 11, 27–32 Search PubMed.
- Larex Inc., GRAS notice, Arabinogalactan from the Eastern Larch Three, 2001 Search PubMed.
- L. Pitkänen and A. M. Striegel, Polysaccharide characterization by hollow-fiber flow field-flow fractionation with on-line multi-angle static light scattering and differential refractometry (HF5/MALS/DRI), J. Chromatogr., A, 2015, 1380, 146–155 CrossRef PubMed.
- G. O. Phillips and P. A. Williams, Handbook of hydrocolloids, CRC Press, Boca Raton, 2000 Search PubMed.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies NDA), Scientific Opinion on the substantiation of health claims related to konjac mannan (glucomannan) and reduction of body weight (ID 854, 1556, 3725), reduction of post-prandial glycaemic responses (ID 1559), maintenance of normal blood glucose concentrations (ID 835, 3724), maintenance of normal (fasting) blood concentrations of triglycerides (ID 3217), maintenance of normal blood cholesterol concentrations (ID 3100, 3217), maintenance of normal bowel function (ID 834, 1557, 3901) and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 1558) pursuant to Article 13(1) of Regulation (EC) No 1924/2006, EFSA J., 2010, 8, 1798 CrossRef.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), D. Turck, J.-L. Bresson, B. Burlingame, T. Dean, S. Fairweather-Tait, M. Heinonen, K. I. Hirsch-Ernst, I. Mangelsdorf, H. J. McArdle, A. Naska, M. Neuhäuser-Berthold, G. Nowicka, K. Pentieva, Y. Sanz, A. Siani, A. Sjödin, M. Stern, D. Tomé, M. Vinceti, P. Willatts, K. Engel, R. Marchelli, A. Pöting, M. Poulsen, J. R. Schlatter, E. Turla and H. Van Loveren, Safety of alginate-konjac-xanthan polysaccharide complex (PGX) as a novel food pursuant to Regulation (EC) No 258/97, EFSA J., 2017, 15, 4776 Search PubMed.
- M. Izydorczyk, S. W. Cui and Q. Wang, in Food Carbohydrates, Chemistry, Physical Properties and Applications, ed. S. W. Cui, CRC Press, Boca Raton, 2005, pp. 263–308 Search PubMed.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), Scientific Opinion on the substantiation of health claims related to guar gum and maintenance of normal blood glucose concentrations (ID 794), increase in satiety (ID 795) and maintenance of normal blood cholesterol concentrations (ID 808) pursuant to Article 13(1) of Regulation (EC) No 1924/2006, EFSA J., 2010, 8, 1464 CrossRef.
- European Commission, definition of Novel Food, http://ec.europa.eu/food/safety/novel_food_en, accessed September 2017.
- European Commission , Regulation (EC) No 2015/2283 of the European Parliament and of the Council, L 371, 2015.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), D. Turck, J.-L. Bresson, B. Burlingame, T. Dean, S. Fairweather-Tait, M. Heinonen, K. Ildico Hirsch-Ernst, I. Mangelsdorf, H. McArdle, A. Naska, M. Neuhauser-Berthold, G. Nowicka, K. Pentieva, Y. Sanz, A. Siani, A. Sjodin, M. Stern, D. Tome, M. Vinceti, P. Willatts, K.-H. Engel, R. Marchelli, A. Pöting, S. Salminen, J. Schlatter, D. Arcella, W. Gelbmann, A. de Sesmaisons-Lecarre, H. Verhagen and H. van Loveren, EFSA J., 2016, 14, 4594–4618 Search PubMed.
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Guidance for submission for food additive evaluations, EFSA J., 2012, 10, 2760 Search PubMed.
- G. Henriksson, in Wood Chemistry and Wood Biotechnology, ed. M. Ek, G. Gellerstedt and G. Henriksson, De Gruyter, Berlin, 2009, pp. 121–145 Search PubMed.
- P. Stenius, Forest Products Chemistry, Fapet Oy, 2000 Search PubMed.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), D. Turck, J.-L. Bresson, B. Burlingame, T. Dean, S. Fairweather-Tait, M. Heinonen, K. I. Hirsch-Ernst, I. Mangelsdorf, H. J. McArdle, A. Naska, M. Neuhäuser-Berthold, G. Nowicka, K. Pentieva, Y. Sanz, A. Siani, A. Sjödin, M. Stern, D. Tomé, M. Vinceti, P. Willatts, K. Engel, R. Marchelli, A. Pöting, M. Poulsen, J. Schlatter, W. Gelbmann and H. Van Loveren, EFSA J., 2017, 15, 4682 Search PubMed.
- European Commission , Commission regulation (EC) No. 1881/2006, Official journal of the European Union, 2006, L364/5-L364/24.
- European Commission, Commission regulation (EC) No. 2015/1006, Official journal of the European Union, 2015, L161/14-L161/16.
- P. O. Kilpeläinen, PhD thesis, Åbo Akademi University, 2015.
- M. Björklund Jansson and N.-O. Nilvebrant, in Wood Chemistry and Wood Biotechnology, ed. M. Ek, G. Gellerstedt and G. Henriksson, De Gruyter, Berlin, 2009, pp. 147–172 Search PubMed.
- M. Ek, G. Gellerstedt and G. Henriksson, Wood Chemistry and Wood Biotechnology, De Gruyter, Berlin, 2009 Search PubMed.
- T. E. Timell, Recent progress in the chemistry of wood hemicelluloses, Wood Sci. Technol., 1967, 1, 45–70 CrossRef CAS.
- M. S. Izydorczyk and C. G. Biliaderis, Cereal arabinoxylans: advances in structure and physiochemical properties, Carbohydr. Polym., 1995, 28, 33–48 CrossRef CAS.
- C. Xu, S. Willför, K. Sundberg, C. Petterson and B. R. Holmbom, Physico-chemical characterization of spruce galactoglucomannan solutions: stability, surface activity and rheology, Cellul. Chem. Technol., 2007, 41, 51–62 CAS.
- C. Xu, R. Korpinen, P. Tuomainen, P. Hirsilä, S. Willför and M. Tenkanen, Abstracts of Papers, 247th ACS National Meeting & Exposition, Dallas, TX, United States, March, 16–20, 2014, CELL-167.
- C. Xu, S. Willför, P. Holmlund and B. Holmbom, Rheological properties of water-soluble spruce O-acetyl galactoglucomannan, Carbohydr. Polym., 2009, 75, 498–504 CrossRef CAS.
- European Commission, Food Safety - Member States support emergency suspension of the sale of jelly-minicups containing “konjac” (E425) food additive, IP/02/435, 2002 Search PubMed.
- C. Xu, A. Pranovich, L. Vähäsalo, J. Hemming, B. Holmbom, H. A. Schols and S. Willför, Kinetics of acid hydrolysis of water-soluble spruce O-acetyl galactoglucomannans, J. Agric. Food Chem., 2008, 56, 2429–2435 CrossRef CAS PubMed.
- M. A. Rao, R. H. Walter and H. J. Cooley, Effect of heat treatment on the flow properties of aqueous guar gum and sodium carboxymethylcellulose (CMC) solutions, J. Food Sci., 1981, 46, 896–899 CrossRef CAS.
- Y. Cheng, K. M. Brown and R. K. Prud'homme, Preparation and characterization of molecular weight fractions of guar galactomannans using acid and enzymatic hydrolysis, Int. J. Biol. Macromol., 2002, 31, 29–35 CrossRef CAS PubMed.
- EFSA, Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to an application on the use of partially depolymerised guar gum as a food additive, EFSA J., 2007, 514, 1–17 Search PubMed.
- M. Rinne, O. Kautto, K. Kuoppala, S. Ahvenjärvi, V. Kitunen, H. Ilvesniemi, S. Willför and R. Sormunen-Cristian, Digestion of wood-based hemicellulose extracts as screened by in vitro gas production method and verified in vivo using sheep, Agric. Food Sci., 2016, 25, 13–21 Search PubMed.
- M. A. Dorato and J. A. Engelhardt, The no-observed-adverse-effect-level in drug safety evaluations: Use, issues, and definition(s), Regul. Toxicol. Pharmacol., 2005, 42, 265–274 CrossRef CAS PubMed.
- World Health Organization, Toxicological evaluation of certain food additives/prepared by forty-sixth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA), 1996.
- National Institute of Health, Carcinogenesis Bioassay of guar gum, NIH publication no 82–1785, 1982 Search PubMed.
- R. Melnick, J. Huff, J. Haseman, M. Dieter, C. Grieshaber, D. Wyand, A. Russfield, A. Murthy, R. Fleischman and H. Lilja, Chronic effects of agar, guar gum, gum arabic, locust bean gum or tara gum in F344 rats and B6C3F1 mice, Food Chem. Toxicol., 1983, 21, 305–311 CrossRef CAS PubMed.
- M. McIvor, C. Cummings and A. Mendeloff, Long-term ingestion of guar gum is not toxic in patients with non insulin-dependent diabetes mellitus, Am. J. Clin. Nutr., 1985, 41, 891–894 CrossRef CAS PubMed.
- EFSA, Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to furfural and furfural diethylacetal, question number EFSA-Q-2003–236, EFSA J., 2004, 67, 1–27 Search PubMed.
- P. M. Jenner, E. C. Hagan, J. M. Taylor, E. L. Cook and O. G. Fitzhugh, Food flavourings and compounds of related structure I. Acute oral toxicity, Food Cosmet. Toxicol., 1964, 2, 327–343 CrossRef CAS.
- E. Boyland, Experiments on the chemotherapy of cancer, Biochem. J., 1940, 34, 1196–1201 CrossRef CAS PubMed.
- G. H. Ayars, L. C. Altman, C. E. Frazier and E. Y. Chi, The toxicity of constituents of cedar and pine woods to pulmonary epithelium, J. Allergy Clin. Immunol., 1989, 83, 610–618 CrossRef CAS PubMed.
- European Parliament and Council Directive No 95/2/EC, Official Journal of the European Communities, 1995, L 61.
- Commission of the European Communities, Reports of the scientific committee for food, 7th edn, 1978 Search PubMed.
- L. Kanerva, O. Tupasela, R. Jolanki, E. Vaheri, T. Estlander and H. Keskinen, Occupational allergic rhinitis from guar gum, Clin. Exp. Allergy, 1988, 18, 245–252 CrossRef CAS.
- F. Lagier, A. Cartier, J. Somer, J. Dolovich and J. Malo, Occupational asthma caused by guar gum, J. Allergy Clin. Immunol., 1990, 85, 785–790 CrossRef CAS PubMed.
- S. Kespohl, N. Kotschy-Lang, J. M. Tomm, M. von Bergen, S. Maryska, T. Brüning and M. Raulf-Heimsoth, Occupational IgE-mediated softwood allergy: characterization of the causative allergen, Int. Arch. Allergy Immunol., 2012, 157, 202–208 CrossRef CAS PubMed.
- K. S. Mikkonen, M. Tenkanen, P. Cooke, C. Xu, H. Rita, S. Willför, B. Holmbom, K. B. Hicks and M. P. Yadav, Mannans as stabilizers of oil-in-water beverage emulsions, LWT–Food Sci. Technol., 2009, 42, 849–855 CrossRef CAS.
- European Commission, Commission regulation (EU) 432/2012, Official Journal of the European Union, 2012, L136/1-L136/40.
- A. Chace Hopkins, T. A. Lehtinen, M. W. Lowe, X. Wang and W. H. Killam Jr., US Pat, US9301540B2, 2016 Search PubMed.
- L. Polari, P. Ojansivu, S. Mäkelä, C. Eckerman, B. Holmbom and S. Salminen, Galactoglucomannan extracted from spruce (Picea abies) as a carbohydrate source for probiotic bacteria, J. Agric. Food Chem., 2012, 60, 11037–11043 CrossRef CAS PubMed.
- Y. Luo, Q. Wang, J. Li, X. Jin and Z. Hao, The relationship between antioxidant activity and total phenolic content in cereals and legumes, Adv. J. Food Sci. Technol., 2015, 8, 173–179 CrossRef CAS.
- S. Willför, M. Ahotupa, J. Hemming, M. Reunanen, P. Eklund, R. Sjöholm, C. Eckerman, S. Pohjamo and B. Holmbom, J. Agric. Food Chem., 2003, 51, 7600–7606 CrossRef PubMed.
- D. Slamenová, J. Lábaj, L. Krizková, G. Kogan, J. Sandula, N. Bresgen and P. Eckl, Protective effects of fungal (1→3)-β-D-glucan derivatives against oxidative DNA lesions in V79 hamster lung cells, Cancer Lett., 2003, 198, 153–160 CrossRef.
- H. Kayali, M. F. Ozdag, S. Kahraman, A. Aydin, E. Gonul, A. Sayal, Z. Odabasi and E. Timurkaynak, The antioxidant effect of β-glucan on oxidative stress status in experimental spinal cord injury in rats, Neurosurg. Rev., 2005, 28, 298–302 CrossRef PubMed.
- T. A. Faber, R. N. Dilger, A. C. Hopkins, N. P. Price and J. G. C. Fahey, The effects of a galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex in broiler chicks challenged with Eimeria acervulina, Poult. Sci., 2012, 91, 1089–1096 CrossRef CAS PubMed.
- J. Rajani, B. Dastar, F. Samadi, M. A. Karimi Torshizi, A. Abdulkhani and S. Esfandyarpour, Effect of extracted galactoglucomannan oligosaccharides from pine wood (Pinus brutia) on Salmonella typhimurium, colonisation, growth performance and intestinal morphology in broiler chicks, Br. Poult. Sci., 2016, 57, 682–692 CAS.
- J. S. Lee, J. S. Kwon, D. P. Won, K. E. Lee, W. C. Shin and E. K. Hong, Study on macrophage activation and structural characteristics of purified polysaccharide from the liquid culture broth of Cordyceps militaris, Carbohydr. Polym., 2010, 82, 982–988 CrossRef CAS.
- J. S. Lee, D. S. Kwon, K. R. Lee, J. M. Park, S. Ha and E. K. Hong, Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth, Carbohydr. Polym., 2015, 120, 29–37 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |