β-Carotene bioaccessibility from biofortified maize (Zea mays) is related to its density and is negatively influenced by lutein and zeaxanthin†
Nivedita
Dube
a,
Purna Chandra
Mashurabad
b,
Firoz
Hossain
c,
Raghu
Pullakhandam
 *b,
Longvah
Thingnganing
d and
Dinesh Kumar
Bharatraj
a
*b,
Longvah
Thingnganing
d and
Dinesh Kumar
Bharatraj
a
aFood and Drug Toxicology Research Center, National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, Telangana, India
bMicronutrient Division, National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, Telangana, India. E-mail: raghu_nin2000@yahoo.com; Fax: +91-40-27019074; Tel: +91-40-27197323
cDepartment of Genetics, Indian Agricultural Research Institute, Indian Council of Agricultural Research, New Delhi, India
dFood Chemistry Division, National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, Telangana, India
First published on 23rd November 2017
Abstract
Biofortification of maize with provitamin A (pro-VA) carotenoids has been successful, but the bioavailability of carotenoids needs to be explored. In the present study, we investigated the carotenoid content, micellarization and intestinal cell uptake of carotenoids from 10 maize hybrids [normal maize, quality protein maize (QPM), pro-VA carotenoid and double biofortified QPM + pro-VA maize hybrids] using a simulated in vitro digestion/Caco-2 cell model. The pro-VA carotenoid content of biofortified maize hybrids is 2–10 fold higher compared to that of normal maize. Furthermore, the ratio of non-pro-VA carotenoids lutein (LUT) plus zeaxanthin (ZEA) to the sum of pro-VA carotenoids β-cryptoxanthin (BCX), α-carotene (AC) and β-carotene (BC) in biofortified maize was much lower compared to that of normal maize. The consumption of 200 g day−1 of biofortified Pusa-PV-16-3 (BC = 808.4 μg per 100 g; AC = 839.3 μg per 100 g; BCX = 59 μg per 100 g) and Pusa-APQH8 (BC = 345.9 μg per 100 g; AC = 1739 μg per 100 g; BCX = 644.2 μg per 100 g) maize would contribute to 52 and 64% of RDAs for adult Indian men, respectively, after adjusting for cooking losses and conversion factors. The mean efficiency of micellarization of LUT (62.2% ± 5.3), ZEA (65% ± 4.7), and BCX (54% ± 9.5) exceeded that of AC (43% ± 8.9) and BC (49.8% ± 7.8) from all the maize hybrids. Furthermore, the micellarization and uptake in Caco-2 cells during a 4 h incubation period showed high correlation (P < 0.05) with the concentration of carotenoids in the maize digesta and micellar fraction, respectively. However, the LUT + ZEA content in the maize digesta and micellar fraction was inversely (p < 0.05) related to the BC micellarization and intestinal cell uptake, respectively. These results together suggest that the enrichment of pro-VA carotenoids together with decreasing the oxygenated carotenoid metabolites such as LUT and ZEA will further improve the bioavailability of BC from maize hybrids.
Introduction
Vitamin A deficiency (VAD) continues to be a major public health problem around the globe leading to stunted growth, blindness and severe infections, particularly in children.1,2 The prevalence of bitot's spots, the clinical sign of VAD, has been on the decline in India over the last four decades.3,4 In contrast, the prevalence of sub-clinical VAD characterized by <20 μg dL−1 serum retinol levels is alarmingly high in both pre-school (61%) and school-age children (55%).5–7 According to the latest report of the National Nutrition Monitoring Bureau,8 the average intake of vitamin A (VA) in 75% Indian rural households was <50% of the RDA (600 μg d−1), therefore, deficits in intakes appear to be a major etiological factor for the observed high prevalence of VAD among children. Therapeutic supplementation and food fortification, apart from advocacy of dietary diversification are being practiced to prevent VAD in the general population. However, poor compliance and the lack of centralized production facilities coupled with household consumption patterns limit the sustainability of these approaches.Typical cereal-based diets do not provide all the essential nutrients, leading to micronutrient malnutrition or ‘hidden hunger’. Therefore, enrichment of nutrients in staple crops through agricultural interventions, viz. agronomy, breeding and biotechnology, collectively referred to as biofortification, is considered a potential approach to mitigate hidden hunger.9 The typical advantages of biofortification are that it ensures a regular daily intake of nutrients across all age groups and once implemented in agricultural systems it is self-sustainable and cost-effective. Based on the available genetic heterogeneity, rice and wheat are being targeted for iron and zinc biofortification, while sweet potato, cassava and maize are being considered for improving provitamin A (pro-VA) carotenoids.10,11 Besides being a critical source of macro- and micronutrients, maize is also a rich source of many phytochemicals, including carotenoids.12 Furthermore, orange maize contains a very high amount of total carotenoids, but xanthophylls (lutein and zeaxanthin) are more abundant in this maize compared to pro-VA carotenoids such as β-carotene.13,14 The development of pro-VA carotenoid-enriched maize has been achieved in the past.15–17 The biofortified maize rich in pro-VA carotenoids was shown to be efficacious in both animal models and human subjects.18–20 Furthermore, the pro-VA enrichment of maize was achieved by the scientists from the Indian Agricultural Research Institute (IARI) via marker-assisted introgression of the crtRB1 allele, and double enrichment by the addition of the recessive opaque2 allele, and the introgression of both crtRB1 and lcyE alleles to provide a high concentration of lysine, tryptophan and pro-VA carotenoids.15 Apart from the density of carotenoids, numerous factors, including the food matrix, dietary fat, dietary fiber, pectin and the competing xanthophylls influence the pro-VA carotenoid bioavailability from plant foods.21–24 Therefore, ensuring the bioavailability of nutrients from biofortified crops is the first necessary nutritional investigation as it provides important clues on the expected efficacy in populations.
In the present study, we investigated the carotenoid content, digestive stability, the efficiency of micellarization, and the intestinal cell uptake of carotenoids from normal and biofortified maize hybrids using a coupled simulated in vitro digestion/Caco-2 cell model. Furthermore, we also analyzed the relationships amongst lutein, zeaxanthin and β-carotene during micellarization and intestinal cell uptake from the maize hybrids. This is the first report of studies on the bioaccessibility of carotenoids in pro-VA rich biofortified maize hybrids developed in India.
Materials and methods
Materials
HPLC grade acetonitrile and dichloromethane, as well as analytical grade petroleum ether, acetone and absolute ethanol, were from Fisher Scientific (Mumbai, India). α-Amylase, pepsin (porcine), pancreatin (porcine), bile extract (porcine), analytical standards of apo-8-carotenal, lutein, zeaxanthin, β-cryptoxanthin, α-carotene and β-carotene and all other chemicals were procured from Sigma Chemical Co. (Bangalore, India).Maize genotypes
The maize genotypes were raised during the years 2015–2016 in a complete randomized design on the research farm of the IARI, New Delhi, India and Maize Research Centre, Professor Jayashankar Telangana State Agricultural University (PJTSAU), Hyderabad, India. The kharif crops were planted at the beginning of the rainy season and harvested in October–November. Post-harvest, the maize kernels were dried under shade without removing the husk. Once dried, the kernels were kept in gunny bags for one month under normal storage conditions. At harvest, 10 genotypes were selected based on the chromatic intensity of kernels and can be broadly divided into four categories, viz. normal maize, Madhuri Sweet Corn (MSC; composite), DHM121 (hybrid) and BML7 (inbred) procured from PJTSAU, Hyderabad, while quality protein maize (QPM) hybrids (HQPM1, Pusa-AQH4), pro-VA biofortified maize hybrids (Pusa-PV-16-3, Pusa-PV-16-2, Pusa-PV-16-4) and double-biofortified QPM + pro-VA maize hybrids (Pusa-APQH8, Pusa-APQH4) were provided by IARI, New Delhi. The pro-VA maize hybrids have an enhanced pro-VA content due to the introgression of the BC hydroxylase (crtRB1) allele through selective breeding.15 While QPM maize hybrids were selectively bred to augment the lysine and tryptophan contents in the kernels by the introgression of the recessive opaque2 allele, QPM + pro-VA maize hybrids were selectively bred (possessing opaque2, crtRB1 and lcyE alleles) for high expression of lysine, tryptophan and pro-VA carotenoids. The handling, extraction, and analysis of all samples were conducted under dim yellow light to minimize photo-oxidative reactions. The representative samples of the maize hybrids were stored at 4 °C upon arrival. The subsamples were ground in a ball mill. The ground samples were placed in 50 mL centrifuge tubes, blanketed with nitrogen, sealed, and stored at −20 °C immediately after grinding until further analysis.All 10 selected maize hybrids were analyzed for carotenoid composition and were subjected to simulated digestion for the estimation of the efficiency of micellerization. Six samples, representative of each maize category, viz. BML7 (normal), Pusa-AQH4 (QPM), Pusa-PV-16-2 and Pusa-PV-16-3 (pro-VA), and Pusa-APQH4 and Pusa-APQH8 (QPM + pro-VA) were used for the estimation of intestinal cell uptake based on their relatively higher carotenoid content, to aid in the detection of carotenoids in cell uptake assays.
Caco-2 cell culture
Colon adenocarcinoma cells (Caco-2) were procured from the American Type Culture Collection (ATCC) and grown as described previously.25 Briefly, the cells, between passages of 28–35, were seeded at a density of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 in 6-well plates and cultured in complete DMEM with 10% fetal bovine serum (FBS, supplemented with 1% NEAA, 0.4 mM glutamine and 1% antibiotic–antimycotic solution) and maintained at 37 °C in an incubator under a 5% CO2/95% air atmosphere at constant humidity.
000 cells per cm2 in 6-well plates and cultured in complete DMEM with 10% fetal bovine serum (FBS, supplemented with 1% NEAA, 0.4 mM glutamine and 1% antibiotic–antimycotic solution) and maintained at 37 °C in an incubator under a 5% CO2/95% air atmosphere at constant humidity.
Preparation of maize porridge
The maize flour (3 g) and peanut oil (3% w/w) were weighed into 50 mL screw cap tubes and 15 mL of distilled water was added to it. The obtained slurry was vortexed vigorously to avoid any lump formation and placed in a boiling water bath set at 95 °C for 15 min. The porridge was cooled to room temperature and subjected to simulated in vitro digestion as described below.Simulated in vitro digestion
The simulated in vitro digestion was carried out as described previously26,27 except that an oral phase of digestion was included and has been described below.Oral phase of digestion
The oral phase of digestion has been included in the study keeping in view the high starch content in maize, as described by Thakkar et al.28 Briefly, the maize porridge was supplemented with solution A (120 mM NaCl, 6 mM CaCl2, and 5 mM KCl) and the volume was made up to 20 mL. The pH of the mixture was adjusted to 7 with 6 N NaOH, followed by the addition of α-amylase (3000 units per g). The tubes were blanketed with nitrogen gas and incubated in a rotating water bath at 37 °C for 10 min.Gastric and intestinal phases of digestion
Briefly, at the end of the oral phase the samples were cooled and the pH was adjusted to 2.5 using 6 N HCl. Pepsin stock solution (in 0.1 M HCl) was added to a final concentration of 2 mg mL−1, and the volume of the solution was increased to 40 mL with solution-A. The mixture was blanketed with nitrogen gas and incubated in a shaking water bath (approx. 60 rpm) for 1 h at 37 °C. At the end of incubation, the pH was raised to 6.5 using 6 N NaOH. The stock solution of bile salts, pancreatin and lipase prepared in 1 M NaHCO3 were then added to the samples to a final concentration of 4.8, 2.8 and 1.4 mg mL−1, respectively. The volume of all the samples was made up to 50 mL with solution-A and incubated in a shaking water bath (60 rpm) for 2 h at 37 °C.Isolation of micellar fraction
Aliquots of the digesta (referred to as ‘digesta’) were transferred into polypropylene quick-seal centrifuge tubes (Beckman Coulter) and centrifuged (Beckman Coulter Optima XL-100 K) at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 477g for 1 h at 4 °C. Post-centrifugation, the aqueous fraction containing mixed micelles was collected and filtered through a 0.22 μM membrane to remove microcrystalline carotenoid aggregates and microbial contamination, if any. The aliquots of digesta and micellar fractions were collected and stored at −20 °C until analysis. Also, a separate aliquot of the micellar fraction was immediately used for studying the intestinal cell uptake in Caco-2 cells, as described below.
477g for 1 h at 4 °C. Post-centrifugation, the aqueous fraction containing mixed micelles was collected and filtered through a 0.22 μM membrane to remove microcrystalline carotenoid aggregates and microbial contamination, if any. The aliquots of digesta and micellar fractions were collected and stored at −20 °C until analysis. Also, a separate aliquot of the micellar fraction was immediately used for studying the intestinal cell uptake in Caco-2 cells, as described below.
Uptake of micellarized carotenoids by Caco-2 cells
The cellular uptake of carotenoids from the aqueous micellar fraction, generated during in vitro digestion, was studied in differentiated Caco-2 cultures, as described previously.27 Briefly, 0.5 mL aliquots of the micellar fraction were immediately diluted with serum free DMEM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and fed to the differentiated Caco-2 cells for a period of 4 h. At the end of incubation, the spent medium was removed; the cell monolayers were washed once with ice-cold phosphate-buffered saline (PBS, pH 7.2) containing 0.5% bovine serum albumin to remove residual carotenoids adhering to the cell surface and twice with only PBS. At the end, the cells were scraped into 1 mL PBS, stored under nitrogen at −20 °C overnight and analyzed the next day.
4) and fed to the differentiated Caco-2 cells for a period of 4 h. At the end of incubation, the spent medium was removed; the cell monolayers were washed once with ice-cold phosphate-buffered saline (PBS, pH 7.2) containing 0.5% bovine serum albumin to remove residual carotenoids adhering to the cell surface and twice with only PBS. At the end, the cells were scraped into 1 mL PBS, stored under nitrogen at −20 °C overnight and analyzed the next day.
Extraction of total carotenoids from maize
The total carotenoids from maize flour were extracted as described previously13 with minor modifications. Briefly, 6 g of maize flour mixed with 30 mL of distilled water was incubated at room temperature for 5 min, followed by addition of 60 mL of absolute ethanol. The samples were then saponified by adding 80% KOH at 85 °C for 10 min. The carotenoids were extracted by using petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone (2
acetone (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and the upper organic phase was collected. The extraction step was repeated thrice and the pooled organic phase was dried under nitrogen and stored at −20 °C until further analysis. The recovery of apo-8 carotenal, used as the internal standard, was consistently high and ranged between 90–95%, therefore the data were not corrected for recovery.
1, v/v), and the upper organic phase was collected. The extraction step was repeated thrice and the pooled organic phase was dried under nitrogen and stored at −20 °C until further analysis. The recovery of apo-8 carotenal, used as the internal standard, was consistently high and ranged between 90–95%, therefore the data were not corrected for recovery.
Extraction of carotenoids from digesta, micellar fraction and cell pellets
The carotenoids from the digesta, micellar fraction and cell pellets were extracted by using petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone (2
acetone (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as described previously.26 Briefly, 3 mL of petroleum ether
1, v/v) as described previously.26 Briefly, 3 mL of petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone (2
acetone (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was added to the sample (digesta and micellar fraction), vortexed for 3 min followed by centrifugation at 5000 rpm for 10 min and the upper organic phase was collected. The cell pellets were thawed and sonicated on ice for 30 seconds after the addition of 1 mL of PBS with 10% ethanol containing 4.5 mmol L−1 butylated hydroxytoluene. The carotenoids were then extracted with 1 mL of petroleum ether
1, v/v) was added to the sample (digesta and micellar fraction), vortexed for 3 min followed by centrifugation at 5000 rpm for 10 min and the upper organic phase was collected. The cell pellets were thawed and sonicated on ice for 30 seconds after the addition of 1 mL of PBS with 10% ethanol containing 4.5 mmol L−1 butylated hydroxytoluene. The carotenoids were then extracted with 1 mL of petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone (2
acetone (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), as mentioned above. The extraction procedure was repeated three times and organic phases were pooled, dried under nitrogen, resolubilized in acetonitrile
1, v/v), as mentioned above. The extraction procedure was repeated three times and organic phases were pooled, dried under nitrogen, resolubilized in acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane (85
dichloromethane (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v) and analyzed by HPLC as described below.
15, v/v) and analyzed by HPLC as described below.
HPLC analysis
The carotenoid HPLC analysis was performed as described previously.26 Briefly, carotenoids were analyzed on an Agilent 1100 HPLC system (Agilent, Model 1100, Puala Alto, CA USA) equipped with an auto-sampler and the UV-Vis detection system controlled by Chemstation software (Version B.02.05). Carotenoids from the total extract, micelles and digesta were fractionated on a reverse-phase column (C-18, 4.6 × 250 mm, Agilent, USA) with a mobile phase (acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane; 85
dichloromethane; 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v) at a flow rate of 1 mL min−1 and monitored at 450 nm. The carotenoids were identified by comparing the retention times of authentic standards and quantified by comparing the area under the curve.
15, v/v) at a flow rate of 1 mL min−1 and monitored at 450 nm. The carotenoids were identified by comparing the retention times of authentic standards and quantified by comparing the area under the curve.
Recommended dietary allowances and computation of retinol equivalents
The Indian recommended dietary allowance (RDA) of vitamin A for adult men is 600 μg retinol equivalents (REs) per day. The REs of the pro-VA carotenoids provided by each maize hybrid after adjusting for cooking losses, with an average daily consumption of 200 g, were computed by considering 1 RE to be equivalent to 8, 16, and 16 μg for BC, AC, and BCX, respectively.29 The percent RDA provided by maize hybrids was computed considering the actual RDA (600 RE) as 100%.Statistical analysis
All experiments were conducted in triplicate and each experiment was repeated twice to provide six independent observations for each maize sample. The digestive stability is the ratio of carotenoids recovered in the digesta after the oral, gastric and intestinal phases of digestion to the carotenoid content in the maize porridge. The percent micellarization is the ratio of carotenoids recovered in the aqueous micellar fraction compared to that of the carotenoid content in digesta. The data were analyzed using SPSS version 21.0 (SPSS, Chicago, IL). Descriptive statistics including mean and SD were calculated for the efficiency of micellarization (%) of carotenoids from digested maize samples. The means were compared using one-way analysis of variance followed by Tukey's t-test. Simple linear regression and Pearson's correlations were performed to test the relationships between the carotenoid micellarization and cellular uptake. The data are considered statistically significant at P < 0.05.Results
Carotenoid content of maize genotypes
Lutein (LUT), zeaxanthin (ZEA), β-cryptoxanthin (BCX), α-carotene (AC) and β-carotene (BC) are the major carotenoids in all the maize hybrids tested (i.e. normal, QPM, pro-VA and QPM + pro-VA maize hybrids). The total carotenoid content before and after the cooking of maize hybrids is given in Table 1. The highest concentration of BC (1036 ± 13.91 μg per 100 g) was found in the pro-VA biofortified Pusa-PV-16-3 maize hybrid while the concentrations of BCX and AC were the highest in the normal hybrid BML7 (1145.99 ± 21.35 μg per 100 g) and double biofortified hybrid Pusa-APQH8 (2046 ± 23.07 μg per 100 g), respectively. The non-pro-VA carotenoid (LUT + ZEA) content was higher in Madhuri sweet corn (1967.3 μg per 100 g) and BML7 (2647.6 μg per 100 g) compared to all other maize hybrids studied. In general, the total pro-VA carotenoid (BCX + AC + BC) content of biofortified maize was 2–10 fold higher compared to normal maize. The average ratio of non-pro-VA to pro-VA carotenoids was higher in normal maize (2.01) compared to that in biofortified maize (0.3). Cooking of maize flour during the preparation of porridge led to 0–24% loss of carotenoids, the greater loss being for AC and BC. Furthermore, the loss of LUT (0–12%), ZEA (2–15%), BCX (0–13%), AC (0–23%) and BC (0–24%) varied among different maize hybrids.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for β-carotene & 16
1 for β-carotene & 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for α-carotene and β-Cryptoxanthin. The percent vitamin A contribution to the RDA from each maize hybrid is computed considering the RDA of 600 μg day−1 for adult men and women, as described in the RDA for Indians, the Indian Council of Medical Research, 2010, India. The values are expressed as the mean ± SD of six independent observations
1 for α-carotene and β-Cryptoxanthin. The percent vitamin A contribution to the RDA from each maize hybrid is computed considering the RDA of 600 μg day−1 for adult men and women, as described in the RDA for Indians, the Indian Council of Medical Research, 2010, India. The values are expressed as the mean ± SD of six independent observations
| Hybrid | Trait | Total content of carotenoids before cooking (μg per 100 g ± SD) | Ratio of non-pro-VA to pro-VA | Total content of carotenoids after cooking (μg per 100 g ± SD) | Total RE from pro-VA μg per 200 g | % RDA per 200 g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lutein | Zeaxanthin | β-Crypto-xanthin | α-Carotene | β-Carotene | Lutein | Zeaxanthin | β-Crypto-xanthin | α-Carotene | β-Carotene | |||||
| MSC | Normal | 1200.4 ± 19.9 | 766.9 ± 16.5 | 285.8 ± 24.0 | 61.9 ± 6.2 | 83.9 ± 7.3 | 4.56 | 1057.3 ± 66.0 | 665.5 ± 39.8 | 250.4 ± 14.8 | 66.4 ± 5.0 | 86.3 ± 4.3 | 61.19 | 10.2 |
| DHM 121 | 329.7 ± 23.7 | 868.8 ± 14.4 | 667.5 ± 29.2 | 27.4 ± 12.6 | 89.5 ± 4.9 | 1.53 | 326.1 ± 20.4 | 777.3 ± 59.0 | 590.7 ± 28.1 | 25.0 ± 1.7 | 91.9 ± 6.0 | 100 | 16.6 | |
| BML7 | 504.5 ± 10.2 | 2143.1 ± 47.0 | 1145.9 ± 21.3 | 41.9 ± 4.6 | 224.8 ± 6.4 | 1.87 | 483.3 ± 26.7 | 1940.2 ± 96.4 | 964.09 ± 110 | 44.3 ± 2.1 | 185.5 ± 15.0 | 172.4 | 28.8 | |
| Pusa-AQH4 | QPM | 459.1 ± 21.0 | 839.0 ± 23.0 | 232.0 ± 13.3 | 474.0 ± 10.6 | 50.5 ± 17.2 | 1.72 | 419.8 ± 26.6 | 780.6 ± 66.2 | 223.4 ± 12.06 | 426.0 ± 33.6 | 44.4 ± 3.5 | 92.3 | 15.3 |
| HQPM1 | 231.0 ± 27.5 | 136.0 ± 11.3 | 76.0 ± 19.2 | 307.0 ± 17.9 | 420.0 ± 18.8 | 0.39 | 228.3 ± 15.9 | 133.5 ± 7.8 | 79.4 ± 1.74 | 277.0 ± 24.8 | 363.8 ± 24.3 | 135.5 | 22.5 | |
| Pusa-PV-16-2 | Pro-VA | 209.3 ± 19.7 | 216.7 ± 32.1 | 61.0 ± 22.2 | 355.0 ± 15.7 | 759.0 ± 10.6 | 0.36 | 210.2 ± 15.0 | 199.2 ± 9.1 | 59.3 ± 3.53 | 313.0 ± 30.5 | 655.1 ± 46.2 | 210.3 | 35.05 |
| Pusa-PV-16-3 | 349.0 ± 11.3 | 166.0 ± 29.5 | 60.0 ± 8.0 | 1084.0 ± 42.4 | 1036.0 ± 13.9 | 0.24 | 332.5 ± 25.1 | 151.7 ± 10.45 | 59.2 ± 4.92 | 839.3 ± 81.3 | 808.4 ± 94.27 | 314.4 | 52.4 | |
| Pusa-PV-16-4 | 152.0 ± 33.1 | 285.0 ± 21.9 | 112.0 ± 18.1 | 414.0 ± 16.9 | 602.0 ± 32.4 | 0.39 | 139.3 ± 8.2 | 276.6 ± 19.3 | 105.76 ± 7.95 | 366.0 ± 25.6 | 475.6 ± 49.3 | 177.8 | 29.6 | |
| Pusa-APQH4 | QPM + Pro-VA | 133.6 ± 38.8 | 272.0 ± 13.7 | 83.0 ± 23.7 | 397.0 ± 34.7 | 766.0 ± 27.5 | 0.33 | 124.6 ± 10.9 | 247.2 ± 11.1 | 88.1 ± 5.81 | 319.5 ± 35.3 | 589.4 ± 62.0 | 198.3 | 33.05 |
| Pusa-APQH8 | 814.0 ± 19.6 | 432.0 ± 11.5 | 752.0 ± 14.4 | 2046.0 ± 23.0 | 414.0 ± 19.7 | 0.39 | 726.4 ± 60.2 | 369.5 ± 29.3 | 644.2 ± 51.13 | 1739 ± 89.0 | 345.9 ± 31.5 | 384.4 | 64.07 | |
The percent contribution of each maize hybrid to the RE and the percent contribution to the RDA of vitamin A are given in Table 1. The consumption of 200 g per day of Pusa-PV-16-3 and Pusa-APQH8 biofortified maize would alone contribute 52% and 64% of the RDA of VA, respectively.
Digestive stability and micellarization of carotenoids during in vitro digestion
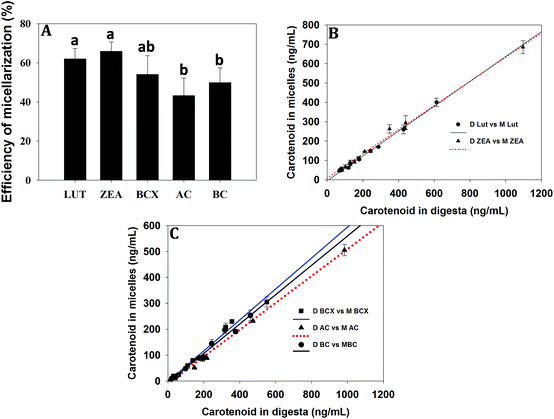
The recovery of non-pro-VA and pro-VA carotenoids after simulated gastrointestinal digestion was always >80% both in normal or biofortified maize hybrids. The mean efficiency of micellarization of LUT (62% ± 5.3), ZEA (65% ± 4.7) and BCX (54% ± 9.5) exceeded that of carotenes (AC 43% ± 8.9; BC 49% ± 7.5) from all the maize hybrids (Fig. 1A). The mean efficiency of micellerization of carotenoids was in the order of LUT = ZEA = BCX ≥ AC = BC. Furthermore, the content of LUT (r = 0.994; p < 0.001), ZEA (r = 0.994; p < 0.001), BCX (r = 0.987; p < 0.001), AC (r = 0.995; p < 0.001) and BC (r = 0.981; p < 0.001) in the aqueous micellar fraction was also significantly correlated with their content in the digesta of the respective maize hybrids (Fig. 1B and C).Uptake of micellarized carotenoids by Caco-2 cells
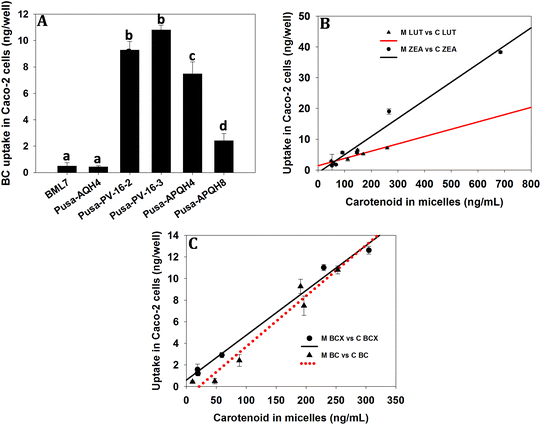
In a comparison of all screened maize hybrids, the BC uptake by Caco-2 cells was significantly higher (P < 0.05) from biofortified maize compared to that from normal maize hybrids (Fig. 2A). Furthermore, the cellular accumulations of LUT (r = 0.808; p < 0.05), ZEA (r = 0.973; p < 0.05), BCX (r = 0.990; p < 0.05) and BC (r = 0.959; p < 0.05) (Fig. 2B and C) were significantly correlated with their quantity present in micelles generated during simulated digestion. In two normal maize hybrids, the cellular uptake of AC was not measurable due to a very low content and therefore, was not analyzed.Effect of lutein and zeaxanthin content on the efficiency of micellarization and the uptake of β-carotene
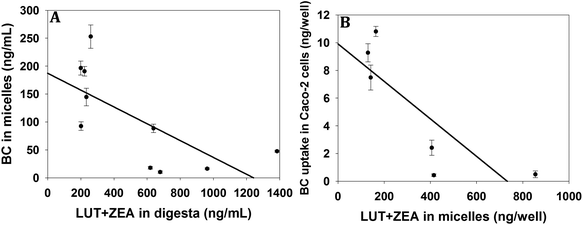
The micellarization of BC was negatively correlated with the content of LUT (r = −0.555; p = 0.09) and ZEA (r = −0.583; p = 0.07) in the digesta, but remained statistically insignificant. However, the BC micellarization was negatively and significantly correlated (r = −0.694; p < 0.05; Fig. 3A) with the LUT + ZEA content in the digesta (Fig. 3A). The cellular uptake of BC was negatively correlated with the content of both ZEA (r = −0.730; p = 0.09) and LUT (r = −0.672; p = 0.14) in the digesta, but remained insignificant (Fig. 3B). But BC uptake is negatively and significantly correlated (r = −0.819; p < 0.05) with the LUT + ZEA content in the digesta (Fig. 3B).Discussion
Biofortification of staple food crops enriched in pro-VA carotenoids is being considered to alleviate the prevalence of VAD in India and many other developing nations.9,11 In addition to the increased nutrient density, ensuring higher bioavailability is required for these foods to be nutritionally superior. The postprandial appearance of carotenoids and retinol in the blood after feeding the test meal in human subjects is an ideal method for measuring the carotenoid bioavailability.22,24,30 However, this method cannot be used as a routine screening tool. Several studies have established that bioaccessibility, as determined by the transfer of carotenoids from the digesta to the aqueous micellar fraction during simulated in vitro digestion, is correlated with bioavailability in humans.30,31In the current study, we evaluated 10 maize hybrids (3-normal, 2-QPM, 3-pro-VA biofortified and 2-double biofortified QPM + pro-VA maize hybrids) with varying carotenoid content for the assessment of in vitro bioaccessibility. The results demonstrate that the pro-VA carotenoid content and micellarization is higher in biofortified maize hybrids compared to normal maize hybrids. Furthermore, the carotenoid content in the micellar fraction remained dependent on its concentration in the digesta. The mean efficiency of micellarization of xanthophylls exceeded that of carotenes in all the maize hybrids. Interestingly, the non-pro-VA carotenoid content in maize hybrids (LUT + ZEA) negatively influenced the micellarization and intestinal cell uptake of BC. These results together suggest that increasing the pro-VA carotenoid content and decreasing the LUT and ZEA will improve the bioavailability of BC from maize hybrids.
The detected carotenoids in the maize hybrids are xanthophylls, viz. LUT, ZEA and BCX, and carotenes, viz. AC and BC, and are consistent with the published results,11,29 except that few investigators did not observe AC in Indian maize hybrids.32–34 The BC concentration was the highest in the pro-VA biofortified hybrid Pusa-PV-16-3 while the non-pro-VA xanthophyll concentration was the highest in the normal maize hybrid samples BML7 and MSC. Furthermore, the non-pro-VA to pro-VA carotenoid ratio was very low in biofortified maize hybrids compared to that in normal maize. Since the biofortification of these maize hybrids was achieved through the introgression of the BC hydroxylase allele (which converts carotenes to xanthophylls via hydroxylation reaction), a decrease in xanthophyll content among biofortified maize hybrids is expected.15 Due to the higher pro-VA carotene content, the biofortified maize hybrids are a potentially significant dietary source of pro-VA carotenoids. Cooking of maize flour during the preparation of porridge led to a 0–24% loss of carotenoids, wherein the loss of LUT, ZEA and BCX was lower (0–15%) compared to that of AC and BC (0–24%). Furthermore, the loss of AC (0–23%) and BC (0–24%) during cooking varied among maize genotypes. Pillay et al.,35 reported a 35% loss of BC during the cooking of maize porridge. However, Mugode et al.36 reported no loss of BC during the preparation of maize porridge or boiling of maize kernels. These studies together suggest a substantial retention of carotenoids in biofortified maize during cooking. The minor differences in the retention of BC during cooking in these studies could be due to the differences in cooking procedures or varietal differences of maize samples. Ortiz et al.37 reported that the carotenoid stability during long-term storage is indeed dependent on the maize genotype. Therefore, further studies are needed to understand the pro-VA carotenoid stability during cooking and associated factors.
The RE is an international standard of measure for VA and is often used to represent the VA activity of pro-VA carotenoids as retinol. ICMR, India has suggested that 8 μg of BC and 16 μg each of AC and BCX from food are required to provide the body with 1 μg of retinol, with a conversion ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 16
1 and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.29 Considering these conversion ratios and the consumption of 200 g of maize per day, the expected REs of cooked biofortified maize Pusa-PV-16-3 and Pusa-APQH8 were 314 and 384, respectively. These REs were equivalent to the 52 and 64% of RDAs for Indian adult men. Therefore, consumption of biofortified maize at the current levels of enrichment may fill the deficits in dietary intake across all age groups.
1, respectively.29 Considering these conversion ratios and the consumption of 200 g of maize per day, the expected REs of cooked biofortified maize Pusa-PV-16-3 and Pusa-APQH8 were 314 and 384, respectively. These REs were equivalent to the 52 and 64% of RDAs for Indian adult men. Therefore, consumption of biofortified maize at the current levels of enrichment may fill the deficits in dietary intake across all age groups.
The carotenoid absorption in intestinal cells requires its release from the food matrix, and the formation of mixed micelles prior to the intestinal cell uptake.22 The high recoveries of carotenoids after the simulated in vitro digestion of maize porridge compared to the initial test material (cooked maize porridge), imply the higher digestive stability of carotenoids. The efficiency of carotenoid micellarization, the ratio of carotenoids transferred to the aqueous micellar fraction to that of its total content in the digesta, represents the bioaccessibility from test foods.27,38,39 The efficiency of micellarization of dietary carotenoids has earlier been reported to be influenced by the type of food matrix, the degree of polarity, and the dietary fat.26,40 Thakkar and Failla41 also reported a higher efficiency of micellarization of xanthophylls compared to that of carotenes from maize. It was suggested that the xanthophylls being polar reside at the surface of lipid droplets and are transferred to a micellar fraction more readily compared to carotenoids, which reside at the core of the lipid droplet.21,42 Furthermore, the food matrix (assuming no changes in the proximate composition during breeding) and dietary fat remained similar in the present study, therefore the observed differences in the magnitude of micellarization among xanthophylls and carotenes could be purely attributed to the polarity of carotenoids. Although the extent of micellarization varied among the carotenoids depending on their degree of polarity, their micellarization significantly correlated with their content in the digesta. Similar trends in the efficiency of micellarization and correlation were also observed with maize samples with varying seed coat color.41 These results imply that increasing the carotenoid content in maize translates to increased micellarization, which in turn would lead to increased bioavailability when fed to human subjects.
The intestinal absorption of carotenoids from mixed micelles is mediated by various proteins, including SR-B1 and NPC1L1.43,44 The carotenoid content, dietary fat composition and other food matrix components such as fiber influence the carotenoid uptake in intestinal cells.22,26,39 However, the intestinal cell uptake of all the carotenoids increased as a function of their concentration in the micellar fraction. In a comparison among the 6 maize hybrids tested for the cellular uptake studies, the BC uptake by Caco-2 cells was significantly higher (P < 0.05) from biofortified maize compared to that from normal maize hybrids. The higher concentration of BC in the micellar fraction and its higher uptake in intestinal cells imply that increasing the pro-VA carotenoid content in the maize translates to increased bioavailability. In agreement with these results, the studies on biofortified cassava also reported that the BC micellarization and intestinal cell uptake remained proportional to its content.28 We also recently demonstrated that the BC uptake in intestinal cells remains proportional to its content in the micellar fraction of digested vegetables and fruits.26
Studies in human subjects have reported that the co-administration of LUT and other oxygenated carotenoids inhibit the BC absorption.45–48 Therefore, LUT and ZEA abundant in certain maize hybrids might interfere with either micellarization and/or the subsequent intestinal cell uptake of BC. Since the non-pro-VA to pro-VA carotenoid ratio varied greatly (4 to 0.3) among maize hybrids used in the present study, it provides an ideal opportunity to investigate the interactive effects, if any. The LUT and ZEA content in the digesta was negatively correlated with the BC micellarization among maize hybrids, though the relationship was not statistically significant. Interestingly, the LUT + ZEA content in the digesta showed significant negative correlation with that of BC micellarization. Tyssandier et al.46 and Borel et al.21 reported that LUT interferes with the transfer of BC to the aqueous micellar fraction. Therefore, the observed negative interactions of LUT and ZEA in BC micellarization could be due to the competition amongst these carotenoids during micellarization. Similarly, the micellar content of LUT and ZEA also showed a negative relationship with the BC uptake in intestinal cells, though the effect was not significant. However, the LUT + ZEA content together was negatively and significantly associated with the BC uptake in intestinal cells (p < 0.05). In agreement with these results, Thakkar et al.41 also demonstrated that LUT interferes with the absorption of BC in intestinal cells when its concentration is a 3-fold molar excess in intestinal Caco-2 cells. Together these results suggest that LUT and ZEA interfere with both the micellarization and intestinal cell uptake of carotenoids from maize and confirm the observations in human studies. Therefore, the biofortification strategy aimed at increasing the carotenoid content via introgression of BC hydroxylase should result in both enhanced pro-VA content and bioavailability.
Conclusion
The results of the present study indicate that biofortified maize with a favorable β-carotene hydroxylase allele enhances the pro-VA carotenoid content and reduces the non-pro-VA xanthophylls. The biofortified Pusa-PV-16-3 and Pusa-APQH8 maize hybrids containing a high pro-VA carotenoid content provide nearly 52% and 64% of RDAs, respectively, at the habitual intake level of 200 g day−1. The carotenoid micellarization and intestinal cell uptake remained dependent on their concentration from all the maize hybrids. Among the individual carotenoids, the efficiency of micellarization is related to their degree of polarity. However, LUT and ZEA appear to exert a negative influence on the BC bioavailability, by interfering with both micellarization and intestinal cell uptake. Therefore, β-carotene hydroxylase allele introgression-based breeding appears to be a promising strategy to increase the content and bioavailability of BC from maize.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr Ravinder Kaur, (past) Director, ICAR- the Indian Agricultural Research Institute (IARI), New Delhi, India and Dr A. K. Singh, Head & Principal Scientist, Division of Genetics, IARI, New Delhi, India for facilitating the study. This work was supported in part by Indian Council of Agricultural Research (ICAR) under the CRP-Biofortification program and the Indian Council of Medical Research (ICMR) in the form of intramural grants. The Caco-2 cell culture facility was established with support from the Department of Biotechnology under a biofortification network. Nivedita Dube and Purna Chandra Mashurabad are supported by research fellowships from the Indian Council for Medical Research and the University Grants Commission, Government of India, respectively.References
- World Health Organization, in Global prevalence of vitamin A deficiency in populations at risk 1995–2005, WHO global database on vitamin A deficiency, World Health Organization, Geneva, Switzerland, 2009, pp. 10–11 Search PubMed.
- M. Lotfi, J. B. Mason, N. Dalmiya, K. Sethuraman and M. Deitchler, Micronutrient report: current progress and trends in the control of vitamin A, iodine, and iron deficiencies, Micronutrient Initiative, IDRC, Ottawa, ON, CA, 2001 Search PubMed.
- G. S. Toteja, P. Singh, B. S. Dhillon and B. N. Saxena, Vitamin A deficiency disorders in 16 districts of India, Indian J. Pediatr., 2002, 69, 603–605 CrossRef CAS PubMed.
- World Health Organization, in Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes, World Health Organization, Geneva, Switzerland, 1996, p. 66 Search PubMed.
- A. Laxmaiah, M. K. Nair, N. Arlappa, P. Raghu, N. Balakrishna, K. M. Rao, C. Galreddy, S. Kumar, M. Ravindranath, V. V. Rao and G. N. Brahmam, Prevalence of ocular signs and subclinical vitamin A deficiency and its determinants among rural pre-school children in India, Public Health Nutr., 2012, 15, 568–577 CrossRef PubMed.
- B. Sivakumar, K. M. Nair, D. Sreeramulu, P. Suryanarayana, P. Ravinder, V. Shatrugna, P. A. Kumar, M. Raghunath, V. V. Rao and N. Balakrishna, Effect of micronutrient supplement on health and nutritional status of schoolchildren: biochemical status, Nutrition, 2006, 22, S15–S25 CrossRef CAS PubMed.
- National Nutrition Monitoring Bureau, Prevalence of micronutrient deficiencies, National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India, 2003 Search PubMed.
- National Nutrition Monitoring Bureau, Diet and nutritional status of population and prevalence of hypertension among adults in rural areas, National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India, 2012 Search PubMed.
- H. Bouis, J. Low, M. McEwan and S. Tanumihardjo, presented in part at the Second International Conference on Nutrition (ICN2), Rome, Italy, November 19–21, 2014.
- F. F. De Moura, A. Miloff and E. Boy, Retention of provitamin A carotenoids in staple crops targeted for biofortification in Africa: cassava, maize and sweet potato, Crit. Rev. Food Sci. Nutr., 2015, 55, 1246–1269 CrossRef CAS PubMed.
- R. M. Welch and R. D. Graham, Breeding for micronutrients in staple food crops from a human nutrition perspective, J. Exp. Bot., 2004, 55, 353–364 CrossRef CAS PubMed.
- E. T. Nuss and S. A. Tanumihardjo, Maize: a paramount staple crop in the context of global nutrition, Compr. Rev. Food Sci. Food Saf., 2010, 9, 417–436 CrossRef CAS.
- A. C. Kurilich and J. A. Juvik, Quantification of Carotenoid and Tocopherol Antioxidants in Zea m ays, J. Agric. Food Chem., 1999, 47, 1948–1955 CrossRef CAS PubMed.
- V. Muthusamy, F. Hossain, N. Thirunavukkarasu, S. Saha, P. Agrawal, S. Guleria and H. Gupta, Genetic variability and inter-relationship of kernel carotenoids among indigenous and exotic maize (Zea mays L.) inbreds, Cereal Res. Commun., 2015, 43, 567–578 CrossRef CAS.
- V. Muthusamy, F. Hossain, N. Thirunavukkarasu, M. Choudhary, S. Saha, J. S. Bhat, B. M. Prasanna and H. S. Gupta, Development of β-carotene rich maize hybrids through marker-assisted introgression of β-carotene hydroxylase allele, PLoS One, 2014, 9, e113583 Search PubMed.
- K. Pixley, N. P. Rojas, R. Babu, R. Mutale, R. Surles and E. Simpungwe, in Carotenoids and human health, ed. S. Tanumihardjo, Springer, 2013, pp. 271–292, DOI:10.1007/978-1-62703-203-2.
- C. E. Harjes, T. R. Rocheford, L. Bai, T. P. Brutnell, C. B. Kandianis, S. G. Sowinski, A. E. Stapleton, R. Vallabhaneni, M. Williams and E. T. Wurtzel, Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification, Science, 2008, 319, 330–333 CrossRef CAS PubMed.
- C. Davis, H. Jing, J. A. Howe, T. Rocheford and S. A. Tanumihardjo, β-Cryptoxanthin from supplements or carotenoid-enhanced maize maintains liver vitamin A in Mongolian gerbils (Meriones unguiculatus) better than or equal to β-carotene supplements, Br. J. Nutr., 2008, 100, 786–793 CrossRef CAS PubMed.
- B. Gannon, C. Kaliwile, S. A. Arscott, S. Schmaelzle, J. Chileshe, N. Kalungwana, M. Mosonda, K. Pixley, C. Masi and S. A. Tanumihardjo, Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial, Am. J. Clin. Nutr., 2014, 100, 1541–1550 CrossRef CAS PubMed.
- S. Li, A. Nugroho, T. Rocheford and W. S. White, Vitamin A equivalence of the β-carotene in β-carotene–biofortified maize porridge consumed by women, Am. J. Clin. Nutr., 2010, 92, 1105–1112 CrossRef CAS PubMed.
- P. Borel, P. Grolier, M. Armand, A. Partier, H. Lafont, D. Lairon and V. Azais-Braesco, Carotenoids in biological emulsions: solubility, surface-to-core distribution, and release from lipid droplets, J. Lipid Res., 1996, 37, 250–261 CAS.
- J. J. Castenmiller and C. E. West, Bioavailability and bioconversion of carotenoids, Annu. Rev. Nutr., 1998, 18, 19–38 CrossRef CAS PubMed.
- J. D. J. Ornelas-Paz, M. L. Failla, E. M. Yahia and A. Gardea-Bejar, Impact of the stage of ripening and dietary fat on in vitro bioaccessibility of β-carotene in ‘Ataulfo'mango, J. Agric. Food Chem., 2008, 56, 1511–1516 CrossRef CAS PubMed.
- K.-J. Yeum and R. M. Russell, Carotenoid bioavailability and bioconversion, Annu. Rev. Nutr., 2002, 22, 483–504 CrossRef CAS PubMed.
- R. Palika, P. C. Mashurabad, S. Kilari, S. Kasula, K. M. Nair and P. Raghu, Citric acid mediates the iron absorption from low molecular weight human milk fractions, J. Agric. Food Chem., 2013, 61, 11151–11157 CrossRef CAS PubMed.
- P. C. Mashurabad, R. Palika, Y. W. Jyrwa, K. Bhaskarachary and R. Pullakhandam, Dietary fat composition, food matrix and relative polarity modulate the micellarization and intestinal uptake of carotenoids from vegetables and fruits, Int. J. Food Sci. Technol., 2017, 1–9 Search PubMed.
- R. Pullakhandam and M. L. Failla, Micellarization and intestinal cell uptake of β-carotene and lutein from drumstick (Moringa oleifera) leaves, J. Med. Food, 2007, 10, 252–257 CrossRef CAS PubMed.
- S. K. Thakkar, B. Maziya-Dixon, A. G. Dixon and M. L. Failla, β-Carotene micellarization during in vitro digestion and uptake by Caco-2 cells is directly proportional to β-carotene content in different genotypes of cassava, J. Nutr., 2007, 137, 2229–2233 CAS.
- ICMR, Nutrient requirements and recommended dietary allowances for Indians: a report of the expert group of the ICMR, National Institute of Nutrition, Hyderabad, 2010 Search PubMed.
- M. L. Failla, T.-Y. Huo and S. K. Thakkar, In vitro screening of relative bioaccessibility of carotenoids from foods, Asia Pac. J. Clin. Nutr., 2008, 17, 200–203 CAS.
- E. Reboul, M. Richelle, E. Perrot, C. Desmoulins-Malezet, V. Pirisi and P. Borel, Bioaccessibility of carotenoids and vitamin E from their main dietary sources, J. Agric. Food Chem., 2006, 54, 8749–8755 CrossRef CAS PubMed.
- M. Vignesh, F. Hossain, T. Nepolean, S. Saha, P. Agrawal, S. Guleria, B. Prasanna and H. Gupta, Genetic variability for kernel β-carotene and utilization of crtRB1 3′TE gene for biofortification in maize (Zea mays L.), Indian J. Genet. Plant Breed., 2012, 72, 189 CAS.
- V. Muthusamy, F. Hossain, N. Thirunavukkarasu, N. Pandey, A. K. Vishwakarma, S. Saha and H. S. Gupta, Molecular characterization of exotic and indigenous maize inbreds for biofortification with kernel carotenoids, Food Biotechnol., 2015, 29, 276–295 CrossRef CAS.
- V. Muthusamy, F. Hossain, N. Thirunavukkarasu, S. Saha, P. Agrawal and H. Gupta, Genetic analyses of kernel carotenoids in novel maize genotypes possessing rare allele of β-carotene hydroxylase gene, Cereal Res. Commun., 2016, 44, 669–680 CrossRef.
- K. Pillay, M. Siwela, J. Derera and F. J. Veldman, Provitamin A carotenoids in biofortified maize and their retention during processing and preparation of South African maize foods, J. Food Sci. Technol., 2014, 51, 634–644 CrossRef CAS PubMed.
- L. Mugode, B. Ha, A. Kaunda, T. Sikombe, S. Phiri, R. Mutale, C. Davis, S. Tanumihardjo and F. F. De Moura, Carotenoid retention of biofortified provitamin A maize (Zea mays L.) after Zambian traditional methods of milling, cooking and storage, J. Agric. Food Chem., 2014, 62, 6317–6325 CrossRef CAS PubMed.
- D. Ortiz, T. Rocheford and M. G. Ferruzzi, Influence of temperature and humidity on the stability of carotenoids in biofortified Maize (Zea mays L.) genotypes during controlled postharvest storage, J. Agric. Food Chem., 2016, 64, 2727–2736 CrossRef CAS PubMed.
- D. A. Garrett, M. L. Failla and R. J. Sarama, Development of an in vitro digestion method to assess carotenoid bioavailability from meals, J. Agric. Food Chem., 1999, 47, 4301–4309 CrossRef CAS PubMed.
- S. R. Goltz, W. W. Campbell, C. Chitchumroonchokchai, M. L. Failla and M. G. Ferruzzi, Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans, Mol. Nutr. Food Res., 2012, 56, 866–877 CAS.
- T. Huo, M. G. Ferruzzi, S. J. Schwartz and M. L. Failla, Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids, J. Agric. Food Chem., 2007, 55, 8950–8957 CrossRef CAS PubMed.
- S. K. Thakkar and M. L. Failla, Bioaccessibility of pro-vitamin A carotenoids is minimally affected by non pro-vitamin A xanthophylls in maize (Zea mays sp.), J. Agric. Food Chem., 2008, 56, 11441–11446 CrossRef CAS PubMed.
- V. Tyssandier, N. Cardinault, C. Caris-Veyrat, M.-J. Amiot, P. Grolier, C. Bouteloup, V. Azais-Braesco and P. Borel, Vegetable-borne lutein, lycopene, and β-carotene compete for incorporation into chylomicrons, with no adverse effect on the medium-term (3-wk) plasma status of carotenoids in humans, Am. J. Clin. Nutr., 2002, 75, 526–534 CAS.
- E. H. Harrison, Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2012, 1821, 70–77 CrossRef CAS PubMed.
- E. Reboul, Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins, Nutrients, 2013, 5, 3563–3581 CrossRef PubMed.
- D. Kostic, W. S. White and J. A. Olson, Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses, Am. J. Clin. Nutr., 1995, 62, 604–610 CAS.
- V. Tyssandier, B. Lyan and P. Borel, Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2001, 1533, 285–292 CrossRef CAS.
- H. van den Berg, Carotenoid interactions, Nutr. Rev., 1999, 57, 1–10 CAS.
- H. van den Berg and T. van Vliet, Effect of simultaneous, single oral doses of beta-carotene with lutein or lycopene on the beta-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men, Am. J. Clin. Nutr., 1998, 68, 82–89 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7fo01034f |
| This journal is © The Royal Society of Chemistry 2018 |