Reshaped fecal gut microbiota composition by the intake of high molecular weight persimmon tannin in normal and high-cholesterol diet-fed rats†
Wei
Zhu
a,
Kuan
Lin
ab,
Kaikai
Li
a,
Xiangyi
Deng
a and
Chunmei
Li
 *ac
*ac
aCollege of Food Science and Technology, Huazhong Agricultural University, Wuhan, 430070, China. E-mail: lichmyl@mail.hzau.edu.cn; Fax: +86-27-87282966; Tel: +86-27-87282966
bWuhan Institutes of Biomedical Sciences, Jianghan University, Wuhan, 430056, China
cKey Laboratory of Environment Correlative Food Science (Huazhong Agricultural University), Ministry of Education, China
First published on 28th November 2017
Abstract
It has been proposed that the gut microbiome may be related to obesity, and diet-induced obesity may induce changes in the gut microbiota composition. Our previous studies suggested that persimmon tannin (PT), which is highly polymerized and non-absorbable in the intestine, showed anti-hyperlipidemic and cholesterol-lowering effects in animal models. Considering that the possible composition modification effects of PT on intestinal bacteria might contribute to its anti-hyperlipidemic and cholesterol-lowering effects in vivo, in this study, we determined whether the PT administration could modify the gut microbiota in both normal diet-fed and high-cholesterol (HC) diet-fed rats, and how PT altered the bacterial composition in both normal and HC fed rats. Sprague-Dawley rats were randomly divided into eight groups, and fed with either a normal or an HC diet supplemented with or without a low/medium/high dose of PT (50 (LPT), 100 (MPT), 200 (HPT) mg per kg body weight (BW) per day, respectively) for 4 weeks. On days 0, 7, 14, 21 and 28, feces were collected and prepared for the microbiota and physicochemical analysis. The results showed that LPT and MPT supplementation significantly altered the gut microbiota composition by increasing the Bacteroidetes/Proteobacteria ratio in both normal diet-fed and HC diet-fed rats. LPT also decreased the Firmicutes/Bacteroidetes ratio in normal diet-fed rats and MPT decreased the Firmicutes/Bacteroidetes ratio in HC diet-fed rats. Both LPT and MPT supplementation induced a significant alteration in specific bacterial species after 14 days of treatment. The relative abundance of Bifidobacterium sp. and Lactobacillus sp. was increased by both LPT and MPT treatment, and that of Escherichia coli and Enterococcus was reduced. Our data also indicate that there is a correlation between the changes in bacterial composition and the changes in short-chain fatty acid (SCFA) metabolism. However, HPT supplementation altered the gut microbiota at the phylum and species levels in an adverse way.
1. Introduction
Obesity, which is considered as one of the major public health issues worldwide, is highly correlated with an increasing incidence of the development of metabolic disorders and many chronic diseases.1,2 Cumulative evidence shows a direct link between intestinal dysbiosis and metabolic diseases, including obesity,3,4 diabetes,5 hepatic disease,6 and cancers.7 Therefore, the regulation of the balance of gut flora could be a potentially effective way for the prevention and treatment of these chronic diseases.Gut microbiota plays a key role in the host physiology and metabolism. It encompasses trillions of bacteria and other microorganisms that live in symbiosis with humans in the gastrointestinal tract. These bacteria can alter the metabolism and immune function and increase fat deposition in the host.8,9 There are over 50 different phyla in the gut microbiota, among which Bacteroidetes and Firmicutes represent the vast majority of the bacterial composition in mice, rats and humans.10,11 Even though there are many bacterial species in the gut, Escherichia coli (E. coli), Bifidobacterium sp., Lactobacillus sp., Bacteroides sp. and Eubacterium sp. are the most predominant bacterial species.12 The gut microbiota is relatively balanced in healthy mice, rats and humans. A high-fat diet alters the gut microbiota physiology in the host, leading to changes in microbiota, such as an increased Firmicutes phylum and decreased Bacteroidetes phylum ratio compared to normal individuals.13,14 Consequently, the long-term imbalance of gut microbiota may result in physiological and metabolic disorders of the host.
Proanthocyanidins are widely distributed in fruits and vegetables such as apples, grapes, persimmons and cranberries, which are commonly consumed in our diet. Proanthocyanidins are reported to exert various bioactivities, such as antioxidant, anticarcinogenic, anti-hyperlipidemic and anti-inflammatory activities.15–18 Epidemiological studies showed that proanthocyanidins and a proanthocyanidin-rich diet can modify the gut microbial composition, thus improving the health of the host.19–21 Tzounis et al. showed that flavan-3-ol monomers such as (−)-epicatechin and (+)-catechin significantly inhibited the growth of Clostridium histolyticum, and enhanced the growth of E. coli in a batch-cultured human large intestine model.22 Proanthocyanidin-rich red wine extract shifted the gut microbiota from a predominance of Bacteroides, Clostridium and Propionibacterium spp. to a predominance of Bacteroides, Lactobacillus and Bifidobacterium spp. (even though the ratio of Bacteroides decreased after the polyphenol treatment, it is still one of the predominant genera, accounting for about 20% of the detected bacterial genera).23 Yamakoshi et al. also documented that a proanthocyanidin-rich grape seed extract significantly increased the Bifidobacteria counts in healthy adults.24 Recently, both in vivo and in vitro studies indicated that the dietary intervention with grape seeds or cocoa extracts could modulate the intestinal microbiota by increasing the counts of beneficial bacteria such as Lactobacillus spp. and inhibiting others such as Clostridium spp.25,26 It is reported that 90–95% of the polyphenols reach the colonic region in a non-absorbable form.27 Therefore, indigested polyphenols could induce composition modifications in intestinal bacteria, which, in turn, might contribute to the potential health benefits of the polyphenols in the host.
Our previous studies demonstrated that persimmon tannin (PT) exerted an excellent anti-hyperlipidemic activity and cholesterol-lowering effect in high-cholesterol (HC) feeding animal models,18 but the detailed underlying mechanisms are still unclear. PT is a type of condensed tannin with a high degree of structural complexity and polymerization, which is not absorbable in the intestine. Considering the direct contact of PT with gut microbiota and the reciprocal interaction between polyphenols and the colonic gut microbial ecosystem,28 possible composition modifications in intestinal bacteria might be attributed to the administration of PT to rats, which might ultimately contribute to the anti-hyperlipidemic and cholesterol-lowering effects in the host. In this study, we used the broad-range sequencing of 16S rRNA from amplified bacterial nucleic acids to investigate the potential effects of PT on the HC diet-induced gut microbiota dysbiosis in rats. Changes in the dominant gut bacteria including Lactobacillus, Bifidobacterium, E. coli, Enterococcus and Clostridium were also evaluated. Meanwhile, we estimated the gut metabolism including the concentrations of different short-chain fatty acids (SCFAs), pH and water content in the rat feces.
2. Materials and methods
2.1 Preparation of PT
Matured and fully colored astringent persimmons (Diospyros kaki Niuxin) were harvested in late October from an orchard in Shanxi province (China). After harvest, the fruits were placed at 100 °C for about 5 min to inactivate polyphenol oxidase, and then deep frozen at −20 °C. PT was prepared according to the method described by Gu et al.29 The content of total polyphenols in PT was determined to be 98.7% by the Folin–Denis method,30 and calculated as gallic acid equivalent. The condensed tannin content in PT was also determined to be 93.4% by acid butanol assay,31 with apple procyanidin dimers as a standard on a mass basis as we previously reported.32The mean polymerization degree of PT was estimated to be 26 by thiolysis degradation combined with HPLC-MS-MS and NMR analysis. The structural characterization and proposed structure are elucidated in our previous report.32 The extension units were determined to be epicatechin, epigallocatechin, (epi) gallocatechin-3-O-gallate, and (epi) catechin-3-O-gallate with relative moles of 2.78, 3.95, 11.0 and 7.58, respectively. The terminal units were determined to be catechin, (epi) gallocatechin-3-O-gallate, and myricetin with relative moles of 0.29, 0.26, and 0.45, respectively.
2.2 Animals and administration
All the experiments were performed in compliance with the Chinese legislation on the use and care of laboratory animals and were approved by the Huazhong Agricultural University of Science and Technology Committee on Animal Care and Use. Forty-eight male Sprague-Dawley rats (120–140 g) were purchased from the Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China). The rats were housed in a temperature and humidity-controlled room (temperature 24 ± 2 °C and humidity 50 ± 10%) with a 12 h light–dark cycle and were given free access to diet and water.After one week of acclimation to the laboratory, the rats were randomly divided into 8 groups, 6 animals each. The 8 groups were designed as follows: group 1 (NC, normal control); group 2 (NC/LPT, normal diet + 50 mg per kg BW (body weight) PT); group 3 (NC/MPT, normal diet + 100 mg per kg BW PT); group 4 (NC/HPT, normal diet + 200 mg per kg BW PT); group 5 (HC, high-cholesterol diet); group 6 (HC/LPT, high-cholesterol diet + 50 mg per kg BW PT); group 7 (HC/MPT, high-cholesterol diet + 100 mg per kg BW PT); group 8 (HC/HPT, high-cholesterol + 200 mg per kg BW PT). The NC group was fed with a basic diet (40% corn, 23% soybean meal, 8% fish meal, 15.5% flour, 6.5% yeast, 4.2% mineral salts, 1.5% oil, 0.8% amino acids, and 0.5% vitamins), while the other groups were fed with a HC diet (81.8% basic diet, 6% dried egg yolk, 5% full cream milk powder, 5% lard, 2% cholesterol, and 0.2% sodiumcholate). Rats in the groups of NC/LPT and HC/LPT were gavaged daily with PT at a dose of 50 mg per kg BW; rats in the groups of NC/MPT and HC/MPT were gavaged daily with 100 mg per kg BW of PT; NC/HPT and HC/HPT were gavaged with 200 mg per kg BW of PT per day, for 4 weeks, meanwhile the rats in group 1 and group 5 were gavaged with the same volume of physiological saline. The rats were given free access to food and water during the experimental period. Food intake and body weight were monitored daily.
The feces were collected into sterile microfuge tubes and then dissolved in sterile physiological saline of 10 times volume (w/v). After the feces became soft and dispersible, the mixture was vortexed for 15 min with a vortex generator, and then stored at −20 °C for further detection of SCFAs, pH and total microbial count. At the end of the experimental period, all animals were fasted for 14 h before sacrifice. The rats were anesthetized with absolute ethyl ether and then sacrificed by cervical vertebral dislocation. Blood samples were drawn from the ophthalmic venous plexus. After centrifugation at 5000g for 15 min at 4 °C, the serum samples were collected and stored at −20 °C. The liver was dissected, rinsed in ice-cold physiological saline, gently blotted on filter paper, weighed and then stored at −20 °C. After laparotomy, the selected parts of the gastrointestinal tract (small intestine, caecum and colon) were removed and weighed. As soon as euthanasia (10 min) was conducted, ileal, cecal and colonic pH values were measured, and the samples of cecum content were taken to determine dry matter and SCFAs. The remaining samples were frozen at −80° C for further 16S rRNA determination.
2.3 Sample analysis
The short-chain fatty acids (SCFAs) concentration in the cecum content was measured by gas chromatography (GC) as previously described with some modifications.33 The cecum contents were weighed, mixed with 0.2 mL of formic acid (internal standard), diluted with deionized water and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min. The supernatant was decanted for GC, for which the conditions were set up as follows: column, DB-FFAP (30 mm × 0.32 mm, i.d. × 0.25 μm); carrier gas, N2, 1.5 mL min−1; column temperature programs, 100 °C (3 min) → 5 °C min−1 → 140 °C (0 min) → 50 °C min−1 → 200 °C (3 min); injection port temperature, 250 °C; detector temperature, 300 °C; detector, FID; injection method, splitless; injection volume, 1 μL.
000g for 5 min. The supernatant was decanted for GC, for which the conditions were set up as follows: column, DB-FFAP (30 mm × 0.32 mm, i.d. × 0.25 μm); carrier gas, N2, 1.5 mL min−1; column temperature programs, 100 °C (3 min) → 5 °C min−1 → 140 °C (0 min) → 50 °C min−1 → 200 °C (3 min); injection port temperature, 250 °C; detector temperature, 300 °C; detector, FID; injection method, splitless; injection volume, 1 μL.
The water content of rats’ feces was determined by the difference between the wet weight of fresh cecum contents and the dry weight of cecum contents which had been dried to a constant mass at 105 °C.
The cecal pH was measured using a microelectrode and a pH/ION meter (model 301, Hanna Instruments, Vila do Conde, Portugal).
2.4 Lipid extraction and analysis from serum and liver
Serum and liver triacylglycerol (TG), total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels were measured using commercially available enzyme kits (Shanghai Mind Bioengineering, Shanghai, China) with a UV-1700 spectrophotometer (Shimadzu, Japan). The centrifuged serum samples can be tested for TG, TC and LDL-C directly using testing kits. The liver (0.5 g) was homogenized in physiological saline (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, w/v) and then lipids were extracted with chloroform
9, w/v) and then lipids were extracted with chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2
methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) overnight. The organic phase was dried under nitrogen and the residue was dissolved in isopropanol and then tested using testing kits.
1, v/v) overnight. The organic phase was dried under nitrogen and the residue was dissolved in isopropanol and then tested using testing kits.
2.5 16S rDNA analysis of gut flora
The cecum samples were analyzed by sequencing the V4 region (16SV4, 233 bp) of 16S ribosomal RNA (rRNA) genes in paired-end modus using the MiSeq system (Illumina, San Diego, CA, USA).DNA was extracted from rat fecal samples using the QIAamp® DNA Stool Mini Kit (Qiagen, Doncaster, Australia). Briefly, a pellet of rat feces was homogenized in lysis buffer and heated at 70 °C. The samples were centrifuged and the supernatant was collected into a centrifuge tube. Inhibitors were adsorbed onto InhibitEX tablets before the samples were centrifuged again and the supernatant was collected. Proteins were digested with proteinase K and buffer, and then DNA was bound to the membrane in the provided spin column. The membrane was washed with wash buffers before DNA was eluted into a centrifuge tube. DNA concentration was determined using a spectrophotometer and diluted to a final working concentration of 1 ng μL−1.
Real-time PCR was performed on a Corbett Rotorgene 3000 rotary cycler (Corbett Research, USA). The primer of 16SV4 used in this study was 515F-806R. PCR mixtures consisted of 1× Quantitect® SYBR Green Master mix (Qiagen), 2.5 ng μL−1 of each primer, and 10 ng of DNA in a volume of 10 μL. The cycling parameters consisted of enzyme activation at 95 °C followed by cycles of melting at 95 °C for 15 s, annealing for the specified times and temperatures, and extension at 72 °C for a specified time. The SYBR green fluorescent signals were acquired at 72 °C. Standard curves were constructed from PCR reactions using 10-fold serial dilutions of known bacterial DNA. The data were analyzed using the Rotor-gene 6 software (Corbett Research).
2.6 Viable count of gut dominant bacteria by cultivation
The feces of rats in all groups were collected and cultivated using selective media for Lactobacillus, Bifidobacterium, E. coli, Enterococcus and Clostridium, respectively. (The selective media are shown in ESI Table S1†). Briefly, about 0.5 g of fresh cecum content was dissolved in 5 mL phosphate buffer solution (PBS, pH7.4). The samples were further shaken, homogenized and diluted to 10−2–10−8 with PBS. After being incubated aerobically or anaerobically at 37 °C for 24 or 48 h, colonies were counted and analyzed from the plates.2.7. Statistical analysis
All data are presented as the mean ± standard deviation (mean ± SD), and evaluated by one-way ANOVA using SPSS (IBM, ver. 19.0), with Duncan's multiple-range test. A P value <0.05 was considered statistically significant.3. Results and discussion
3.1 The effects of PT on the serum, liver lipid and organ weight of rats fed with a high-cholesterol diet
After 4 weeks of the dietary treatments, 3 normal diet groups (NC/LPT, NC/MPT and NC/HPT) and 4 high-cholesterol diet groups (HC, HC/LPT, HC/MPT and HC/HPT) had similar body weights and food intake to the NC group (data are shown in ESI Table S2†). The serum TG, TC and LDL-C of the HC diet groups were significantly higher (p < 0.05) than those of the NC groups (Table 1). Nevertheless, PT administration for 4 weeks significantly reduced the TG, TC and LDL-C levels in the 3 HC-groups. Compared with the HC group alone, LPT, MPT, and HPT treatment reduced the TG levels by 28.6, 39.0, and 25.6%, TC levels by 26.9, 22.8, and 20.8% and LDL-C levels by 25.2, 11.6, and 4.0%, respectively. The liver TG and TC levels of the HC group were also increased significantly (p < 0.05) compared to the NC group, and the PT administration decreased the TG and TC levels in the liver. These results confirmed the anti-hyperlipidemic effects of PT in vivo.| NC | HC | HC/LPT | HC/MPT | HC/HPT | |
|---|---|---|---|---|---|
| TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol. NC, normal control group; NC/LPT, normal control with a low dose (50 mg per kg body weight) of the persimmon tannin group; NC/MPT, normal control with a moderate dose (100 mg per kg body weight) of the persimmon tannin group; NC/HPT, normal control with a high dose (200 mg per kg body weight) of the persimmon tannin group; HC, high-cholesterol group; HC/LPT, high-cholesterol with a low dose (50 mg per kg body weight) of the persimmon tannin group; HC/MPT, high-cholesterol with a moderate dose (100 mg per kg body weight) of the persimmon tannin group; HC/HPT, high-cholesterol with high dose (200 mg per kg body weight) of the persimmon tannin group. The results are expressed as mean ± S.E.M. (n = 6). Values in the columns marked with * indicate a significant difference (p < 0.05) versus NC group and # indicates a significant difference (p < 0.05) versus HC group. | |||||
| Serum (μmol L −1 ) | |||||
| TG | 264.99 ± 25.70 | 596.89 ± 44.14* | 429.22 ± 36.03* | 363.81 ± 24.77# | 444.20 ± 31.51* |
| TC | 135.96 ± 15.08 | 346.56 ± 13.74* | 253.23 ± 10.19*# | 267.55 ± 16.62* | 274.40 ± 15.55*# |
| LDL-C | 515.61 ± 34.57 | 762.04 ± 24.74* | 569.75 ± 17.08# | 673.36 ± 48.72 | 728.90 ± 36.76* |
| Liver (μmol L −1 ) | |||||
| TG | 50.97 ± 4.00 | 77.70 ± 5.94* | 73.42 ± 4.84* | 67.03 ± 3.00 | 70.52 ± 0.21* |
| TC | 25.66 ± 1.09 | 32.21 ± 4.38 | 36.75 ± 7.45 | 38.01 ± 2.70* | 37.72 ± 3.31* |
| Weight (g) | |||||
| Liver | 17.04 ± 1.13 | 16.91 ± 1.99 | 15.72 ± 1.54 | 16.42 ± 0.71 | 18.30 ± 1.11 |
| Heart | 1.11 ± 0.08 | 1.03 ± 0.13 | 1.05 ± 0.16 | 1.16 ± 0.05 | 1.35 ± 0.18*# |
| Kidney | 2.80 ± 0.20 | 2.43 ± 0.27 | 2.42 ± 0.45 | 2.64 ± 0.20 | 2.58 ± 0.38 |
| Spleen | 0.81 ± 0.08 | 0.72 ± 0.02 | 0.77 ± 0.20 | 0.78 ± 0.07 | 0.92 ± 0.21 |
3.2 The effects of PT on gut microbiota at the phylum level by 16S rDNA
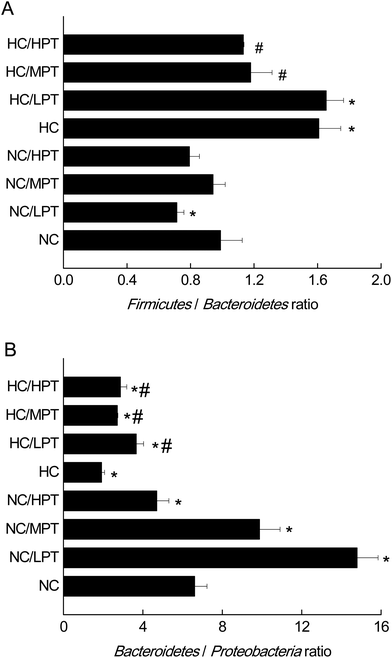
At the phylum level, LPT and MPT supplementation caused significant alteration of Firmicutes (−17%, p < 0.05) and Bacteroidetes (+18%, p < 0.05) in normal rats, and the diet with HPT significantly increased Proteobacteria (+44%, p < 0.01) (Table 2). Moreover, a significant decrease (−30%, p < 0.001) was detected in the Firmicutes/Bacteroidetes ratio of normal diet rats supplemented with LPT, which was not observed in MPT and HPT supplementation groups (Fig. 1A). In addition, a significant rise of the Bacteroidetes/Proteobacteria ratio was detected in NC groups supplemented with LPT (+123%, p < 0.001) and MPT (+54%, p < 0.001), separately. In contrast, a significant decrease (−44%, p < 0.001) of Bacteroidetes/Proteobacteria ratio was observed in the NC group supplemented with HPT (Fig. 1B). Compared with the NC diet, the HC diet caused a significant decrease of Bacteroidetes (−28%, p < 0.01) and a significant increase of Proteobacteria phylum (+62%, p < 0.001) (Table 2). Meanwhile, a significant increase of Firmicutes/Bacteroidetes ratio (+40%, p < 0.001) and a significant decrease of Bacteroidetes/Proteobacteria ratio (−69%, p < 0.001) were observed in the HC group compared with the NC group. Our results agreed with the previous studies11,34 in which a high-fat diet induced microbial composition alteration, usually associated with an increased Firmicutes/Bacteroidetes ratio and a decreased Bacteroidetes/Proteobacteria ratio. However, HC-diet rats supplemented with LPT and MPT showed a significant (p < 0.01 vs. HC group) reduction in the abundance of the Firmicutes phylum, which commonly dominates in obesity and overweight status.35 Supplementation with MPT or HPT significantly (p < 0.001) decreased the Firmicutes/Bacteroidetes ratio by about 30%, compared to the HC group (Fig. 1A). Many in vivo studies suggested that polyphenols might alter the gut microbial composition at the phylum level in HC-diet rats or humans.19,20,36 For example, Etxeberria et al. reported that administration of 30 mg per kg BW per day of quercetin to the high-fat-sugar diet rats resulted in a prominent decrease (−80%) in Firmicutes/Bacteroidetes ratio, and a significant reduction (−34%) in the Firmicutes phylum, while the bacterial composition of the feces from the groups supplemented with trans-resveratrol was not significantly affected at the phylum level.36 Meanwhile, the three HC diet groups supplemented with PT showed a significantly (p < 0.01) higher Bacteroidetes/Proteobacteria ratio compared to the individual HC group (Fig. 1B) as the ratio was increased by 90% with LPT supplementation, and by about 60% with both MPT and HPT supplementation. These data demonstrated that LPT altered the gut microbial composition significantly by decreasing the Firmicutes/Bacteroidetes ratio (p < 0.01) and increasing the Bacteroidetes/Proteobacteria ratio in normal diet rats. The HC diet induced a significantly higher Firmicutes/Bacteroidetes ratio and lower Bacteroidetes/Proteobacteria ratio compared to normal diet rats (p < 0.01), while MPT and HPT supplementation could prevent the HC diet-induced gut microbiota change to some extent. When comparing the effect of PT on the gut microbiota at the phylum level between NC and HC groups, we found that the effect of PT on the HC-diet animals was less effective than that on the normal rats, with the Bacteroidetes/Proteobacteria phylum ratios of the HC/PT groups being between 2 and 4, which were lower than the ratios of the control groups (between 6 and15).| Firmicutes | Bacteroidetes | Proteobacteria | |
|---|---|---|---|
| NC, normal control group; NC/LPT, normal control with a low dose (50 mg per kg body weight) of the persimmon tannin group; NC/MPT, normal control with a moderate dose (100 mg per kg body weight) of the persimmon tannin group; NC/HPT, normal control with a high dose (200 mg per kg body weight) of the persimmon tannin group; HC, high-cholesterol group; HC/LPT, high-cholesterol with a low dose (50 mg per kg body weight) of the persimmon tannin group; HC/MPT, high-cholesterol with a moderate dose (100 mg per kg body weight) of the persimmon tannin group; HC/HPT, high-cholesterol with a high dose (200 mg per kg body weight) of the persimmon tannin group. The results are expressed as mean ± S.E.M. (n = 6). Values in the columns marked with * indicate a significant difference (p < 0.05) versus NC group and # indicates a significant difference (p < 0.05) versus HC group. | |||
| NC | 44.41 ± 3.33 | 48.66 ± 4.20 | 5.92 ± 0.49 |
| NC/LPT | 36.69 ± 3.06* | 57.65 ± 5.15* | 4.42 ± 0.45 |
| NC/MPT | 41.99 ± 4.38 | 45.13 ± 4.07 | 6.32 ± 0.64 |
| NC/HPT | 37.07 ± 1.92* | 50.07 ± 5.14 | 10.59 ± 0.78* |
| HC | 44.31 ± 2.32 | 35.04 ± 2.97* | 15.73 ± 1.48* |
| HC/LPT | 50.11 ± 3.74 | 34.17 ± 2.18* | 12.40 ± 1.21* |
| HC/MPT | 49.24 ± 1.73 | 33.81 ± 3.83* | 13.30 ± 1.44* |
| HC/HPT | 43.39 ± 1.70 | 37.87 ± 2.12 | 15.30 ± 1.15* |
3.3 The effects of PT on the gut microbiota at the genus level
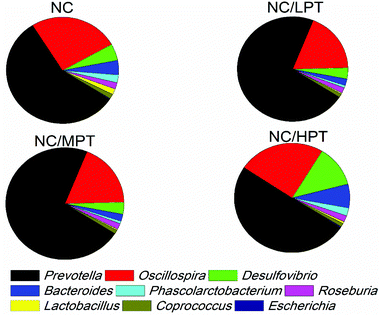
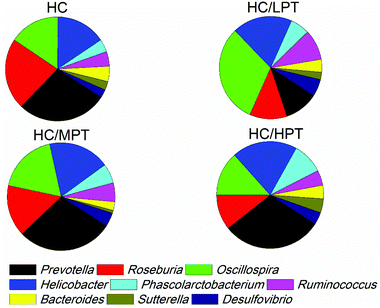
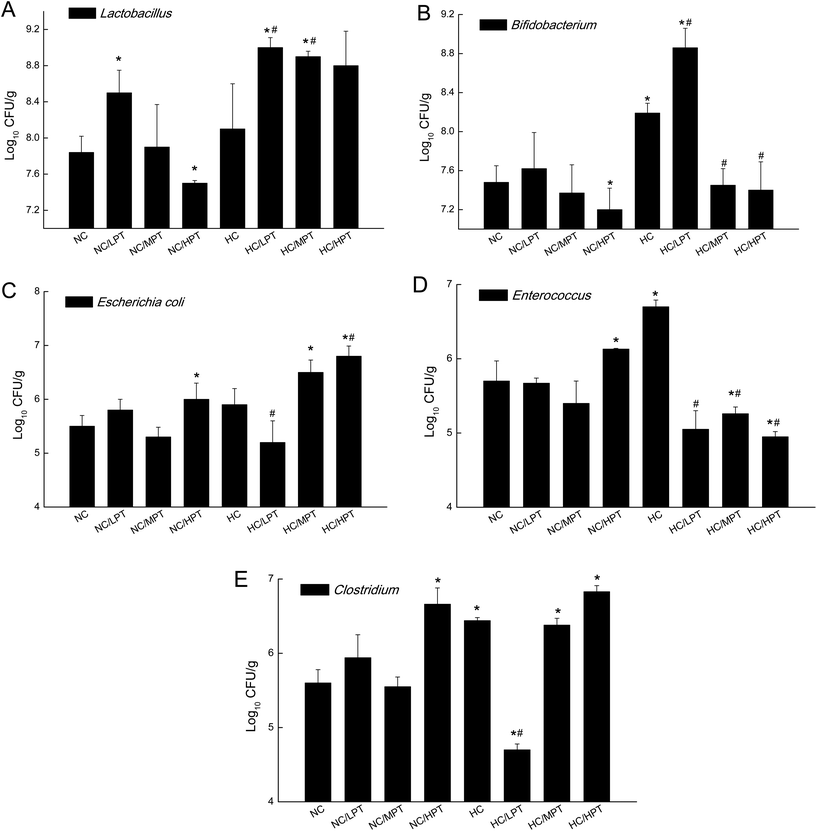
Regarding the genus level, the bacterial composition in different groups also varied. In normal diet-fed rats, groups supplemented with LPT showed a significantly different fecal bacterial profile from that of the NC group. The LPT supplementation increased the Prevotella genus by 38% (p < 0.001), but it produced a significant inhibition on Bacteroides (−40%, p < 0.001), Phascolarctobacterium (−60%, p < 0.001) and E. coli (−23%, p < 0.05), compared to the NC group (Fig. 2). In contrast, MPT and HPT supplementation displayed the opposite effect. We also provided the relative abundance (% of total 16S rDNA) of the most representative genus in normal diet rats (Table S3†). In the HC group, the bacterial composition was significantly different from that of the NC group. The beneficial Prevotella was reduced by 56% (p < 0.001), while the harmful Roseburia, Phascolarctobacterium and Helicobater were significantly increased by about 90%, 50% and 66% (p < 0.001), respectively. Ruminococcus, which belongs to the Firmicutes phylum, was also increased in the HC group, while it was hardly detected in the NC group. Meanwhile, the HC diet influenced the balance of gut microbiota through inhibiting the growth of beneficial bacteria and promoting the growth of harmful ones. The microbiota composition-altering effect of the HC diet was probably improved by LPT supplementation. Roseburia and Helicobater were significantly decreased by 63% (p < 0.001) and 16% (p < 0.05), respectively (Fig. 3). The relative abundance (% of total 16S rDNA) of the most representative genus in HC diet rats is also shown in Table S4.†We also determined the profile of the predominant gut microorganisms, including Lactobacillus, Bifidobacterium, E. coli, Enterococcus and Clostridium, which mostly contribute to the intestinal micro-ecological balance. The colony forming unit in the feces on day 28 expressed as log10 values are shown in Fig. 4. In normal diet-fed rats, LPT supplementation significantly (p < 0.05) increased the total Lactobacillus count, and MPT led to a slight increase (p > 0.05). On the other hand, HPT supplementation significantly (p < 0.05) reduced the count of Lactobacillus. For another beneficial bacterium Bifidobacterium, no significant difference between the NC group and the LPT administration group was observed, but MPT and HPT treatment led to a significant decrease of Bifidobacterium (p < 0.05). In terms of harmful bacteria, LPT and MPT supplementation did not influence the counts of E. coli, Enterococcus and Clostridium, which were, however, significantly (p < 0.05) increased by HPT. These results were consistent with the results of 16S rDNA, implying that LPT and MPT could promote the growth of the beneficial bacterium such as Lactobacillus, but HPT could inhibit the growth of beneficial bacteria Lactobacillus and Bifidobacterium, and increase the harmful ones such as E. coli, Enterococcus and Clostridium. Compared to the NC diet, the HC diet increased the viable count of Bifidobacterium, E. coli and Enterococcus significantly (p < 0.05), but not the total count of Lactobacillus. However, the HC diet supplemented with LPT or MPT for 4 weeks induced a significant (p < 0.05) increase in the viable count of Lactobacillus (Fig. 4A). LPT supplementation increased the growth of Bifidobacterium significantly (p < 0.05), but MPT and HPT supplementation exerted opposite effects (Fig. 4B). When comparing the effects of different doses of PT on the harmful bacteria E. coli and Enterococcus, we found that LPT supplementation inhibited their growth induced by the HC diet significantly (p < 0.05). MPT and HPT supplementation also displayed significant inhibiting effects (p < 0.05 vs. NC and HC groups) on Enterococcus, whereas they promoted the growth of E. coli (Fig. 4C and D). Different doses of PT had dissimilar effects against Clostridium and E. coli, with LPT suppressing but MPT and/or HPT encouraging their growth (Fig. 4E).
Compared to normal diet-fed rats, species such as E. coli, Enterococcus and Clostridium significantly (p < 0.01) thrived in HC-diet fed rats. Lactobacillus increased by about 5% unexpectedly in HC diet rats, which was consistent with the result reported by H. Daniel et al.,11 but diet-associated differences were statistically insignificant (p > 0.05), suggesting that the slight increase of Lactobacillus in the HC group may be due to marked inter-individual variations. An animal study carried out by Smith et al.37 found that after rats were given a tannin-rich diet, the Bacteroides group increased significantly, while the Clostridium leptum cluster decreased significantly. Our results showed that MPT also significantly increased Bacteroides and decreased the Clostridium genus in normal diet rats, which was consistent with the result of previous studies. Dolara et al. reported that when the rats were treated with red-wine polyphenols (50 mg per kg BW), they showed a significantly lower level of Clostridium spp. and enhanced levels of Bacteroides, Bifidobacterium and Lactobacillus spp.23 The present results are in line with the results of previous studies.
The previous study reported that an excessive polyphenol intake would be deleterious for the host, including their pro-oxidant activity, mitochondrial toxicity (potential apoptosis-inducing properties), and interactions with drug-metabolizing enzymes.38 As for HPT (200 mg per kg BW supplementation), it decreased the Bacteroidetes/Proteobacteria ratio significantly (p < 0.05), and in the meantime, it reduced the beneficial Bifidobacterium, but increased the harmful E. coli and Clostridium significantly (p < 0.05). The opposite effects of HPT implied that a high concentration of PT could possibly influence the gut bacterial balance adversely, which might be harmful to the rats. Therefore, due to the potential risks, the appropriate amount of dietary flavonoid/phenolic consumption or exposure would be important. In the present study, the dose of 200 mg per kg BW per day PT in the rats corresponds to 2.2 g of PT for a healthy adult (BW = 70 kg), which is equivalent to consuming 3–4 persimmon fruits (medium, 200 g) per day for an adult. However, it was unlikely to have such a high intake of persimmon fruits every day.
3.4 The effects of persimmon tannin on gastrointestinal metabolism
| Acetic acid | Propionic acid | n-Butyric acid | Isobutyric acid | Pentanoic acid | Total | |
|---|---|---|---|---|---|---|
| SCFAs, short-chain fatty acids; NC, normal control group; NC/LPT, normal control with a low dose (50 mg per kg body weight) of the persimmon tannin group; NC/MPT, normal control with a moderate dose (100 mg per kg body weight) of the persimmon tannin group; NC/HPT, normal control with a high dose (200 mg per kg body weight) of the persimmon tannin group; HC, high-cholesterol group; HC/LPT, high-cholesterol with a low dose (50 mg per kg body weight) of the persimmon tannin group; HC/MPT, high-cholesterol with a moderate dose (100 mg per kg body weight) of the persimmon tannin group; HC/HPT, high-cholesterol with a high dose (200 mg per kg body weight) of the persimmon tannin group. The results are expressed as mean ± S.E.M. (n = 6). Values in the columns marked with * indicate a significant difference (p < 0.05) versus NC group and # indicates a significant difference (p < 0.05) versus HC group. | ||||||
| NC | 35.58 ± 3.42 | 24.59 ± 1.87 | 4.74 ± 0.02 | 26.2 ± 2.19 | 5.71 ± 0.55 | 96.82 ± 7.18 |
| NC/LPT | 91.74 ± 5.79* | 77.60 ± 7.65* | 3.05 ± 0.29 | 33.31 ± 3.02 | 20.07 ± 1.66* | 225.77 ± 16.13* |
| NC/MPT | 107.05 ± 8.68* | 120.65 ± 12.33* | 8.9 ± 0.76* | 62.61 ± 6.03* | 24.98 ± 1.01* | 324.21 ± 17.71* |
| NC/HPT | 80.50 ± 7.90* | 43.96 ± 4.73* | 1.3 ± 0.13* | 6.33 ± 0.37* | 6.83 ± 0.74 | 138.93 ± 11.14* |
| HC | 16.45 ± 0.86* | 18.96 ± 1.54* | 2.56 ± 0.09* | 6.42 ± 0.52* | 4.75 ± 0.90 | 49.14 ± 3.88* |
| HC/LPT | 54.71 ± 5.09# | 28.20 ± 2.87# | 5.72 ± 0.36# | 26.37 ± 1.98# | 8.4 ± 1.02# | 123.44 ± 8.19*# |
| HC/MPT | 40.27 ± 3.32# | 4.93 ± 0.32# | 7.17 ± 0.44# | 6.34 ± 0.78# | 5.47 ± 0.53 | 64.38 ± 5.72 |
| HC/HPT | 28.20 ± 2.33 | 15.14 ± 1.84 | 1.47 ± 0.16 | 16.14 ± 1.84 | 1.51 ± 0.10 | 62.46 ± 6.14 |
SCFAs are the major metabolites of gut microbiota. Analysis of the SCFAs profile clearly demonstrated that PT administration affected the count and metabolic activity of the bacteria present in normal diet-fed rats and HC-diet fed rats. In NC groups, LPT and MPT supplementation significantly (p < 0.01) increased the levels of acetic, propionic and pentanoic acids, resulting in a sharp increase in the total cecum SCFAs. It was reported that propionic acid was produced mainly by Bacteroides, while acetic acid and butyric acid were generated mostly by Clostridium.39 LPT and MPT supplementation notably increased the amount of Lactobacillus, Bifidobacterium and Clostridium (Fig. 1), thus resulting in higher levels of acetic acid and propionic acid in NC groups. The impaired microbial metabolic activity corresponded to the changes in microbial composition and dominant bacterial amounts at the end of the PT treatment. The HC-diet led to a sharp decrease of all SCFAs concentrations except for pentanoic acid, but the LPT administration significantly (p < 0.05) increased all SCFAs concentrations. Different from our results, Etxeberria et al.36 reported that supplementing high fat-diet-fed rats with trans-resveratrol or quercetin significantly altered the bacterial compositions, but slightly changed the SCFAs profile in the large intestine. However, Cowan et al. documented that chronic coffee consumption in diet-induced obese rats resulted in a notable increase of SCFAs,40 which was consistent with our results.
| pH | Water content (g g−1 feces) | |
|---|---|---|
| NC, normal control group; NC/LPT, normal control with a low dose (50 mg per kg body weight) of the persimmon tannin group; NC/MPT, normal control with a moderate dose (100 mg per kg body weight) of the persimmon tannin group; NC/HPT, normal control with a high dose (200 mg per kg body weight) of the persimmon tannin group; HC, high-cholesterol group; HC/LPT, high-cholesterol with a low dose (50 mg per kg body weight) of the persimmon tannin group; HC/MPT, high-cholesterol with a moderate dose (100 mg per kg body weight) of the persimmon tannin group; HC/HPT, high-cholesterol with a high dose (200 mg per kg body weight) of the persimmon tannin group. The results are expressed as mean ± S.E.M. (n = 6). Values in the columns marked with * indicate a significant difference (p < 0.05) versus NC group and # indicates a significant difference (p < 0.05) versus HC group. | ||
| NC | 7.30 ± 0.52 | 0.29 ± 0.03 |
| NC/LPT | 6.40 ± 0.03* | 0.20 ± 0.01* |
| NC/MPT | 6.62 ± 0.08* | 0.39 ± 0.02* |
| NC/HPT | 6.71 ± 0.06 | 0.17 ± 0.02* |
| HC | 5.96 ± 0.04* | 0.18 ± 0.01* |
| HC/LPT | 5.94 ± 0.22* | 0.39 ± 0.02*# |
| HC/MPT | 6.36 ± 0.03*# | 0.36 ± 0.02*# |
| HC/HPT | 5.86 ± 0.07* | 0.26 ± 0.02# |
The water content is also a crucial indicator of intestinal balance. When the intestinal balance is disrupted, the water content in the feces will be changed. As shown in Table 4, LPT supplementation exerted no significant alteration in the water content in the cecum content, while MPT supplementation caused a significant (p < 0.05) increase, and HPT supplementation caused a significant (p < 0.05) decrease of water content in normal diet rats. The HC diet lowered the water content in the feces by 40% (p < 0.05) compared to the normal diet rats, while PT supplementation partially prevented the water-lowering effect of the HC diet on the feces.
It is acknowledged that natural polyphenols have wide biological properties. Previous studies from our laboratory and other groups strongly indicated that PT was associated with various health-promoting effects such as anti-oxidant, anti-obese, cholesterol-lowering, and liver-protecting effects.18,41–43 The health outcomes of polyphenols are intimately dependent on the dose, supplying form and bioavailability within the organism.44 Nevertheless, as a highly polymerized condensed tannin, PT can hardly be absorbed in the intestine. Given the gut microbiota and metabolism, polyphenols may be converted to bioactive compounds that can affect the intestinal ecology and influence host health by the colonic microbiota. Increasing evidence from both animal and human studies suggested that certain doses of selected polyphenols may influence host health by altering the gut microbial composition.14,19,20,45 In view of the effects of PT on the gut microbiota in both normal diet-fed rats and HC-diet fed rats in the present study, we proposed that the modified composition of intestinal bacteria by PT might ultimately contribute to its biological activity in vivo.
4. Conclusions
In summary, LPT and MPT supplementation significantly altered the gut microbiota composition by increasing the Bacteroidetes/Proteobacteria ratio both in normal diet-fed rats and HC-diet fed rats. LPT supplementation also decreased the Firmicutes/Bacteroidetes ratio in normal diet-fed rats and MPT supplementation decreased the Firmicutes/Bacteroidetes ratio in HC-diet fed rats. Furthermore, supplementation with LPT induced a significant alteration in specific bacterial species by increasing some types of gut microbiota that have been inversely related to obesity (Bifidobacterium sp., Lactobacillus sp. ) and reducing the relative abundance of others associated with diet-induced obesity (E. coli and Enterococcus). Our results suggested that the hypolipidemic effect of PT in vivo might be partly attributed to its significant improvement of gut microbiota composition altered by the HC diet. The intestinal bacterial composition modifying effects of LPT and MPT supplementation might initially contribute to their proposed health benefits in vivo. HPT supplementation exerted an adverse effect on gut bacteria at both phylum and species levels.Conflicts of interest
The authors have declared no conflicts of interest.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31571839) and the Special Fund for Agro-scientific Research in the Public Interest (No. 201203047). We thank Dr Ziwei Wang in the University of California-Davis for her contribution to the English language revise of this paper.References
- M. M. Finucane, G. A. Stevens, M. J. Cowan, G. Danaei, J. K. Lin, C. J. Paciorek, G. M. Singh, H. R. Gutierrez, Y. Lu, A. N. Bahalim, F. Farzadfar, L. M. Riley and M. Ezzati, Lancet, 2011, 377, 557–567 CrossRef.
- P. G. Kopelman, Nature, 2000, 404, 635–643 CrossRef CAS PubMed.
- S. Guida and K. Venema, J. Funct. Foods, 2015, 14, 407–423 CrossRef CAS.
- P. J. Turnbaugh, R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis and J. I. Gordon, Nature, 2006, 444, 1027–1031 CrossRef PubMed.
- P. D. Cani, R. Bibiloni, C. Knauf, A. Waget, A. M. Neyrinck, N. M. Delzenne and R. Burcelin, Diabetes, 2008, 57, 1470 CrossRef CAS PubMed.
- A. Goel, M. Gupta and R. Aggarwal, J. Gastroenterol. Hepatol., 2014, 29, 1139–1148 CrossRef PubMed.
- H. Tlaskalova-Hogenova, R. Stepankova, H. Kozakova, T. Hudcovic, L. Vannucci, L. Tuckova, P. Rossmann, T. Hrncir, M. Kverka, Z. Zakostelska, K. Klimesova, J. Pribylova, J. Bartova, D. Sanchez, P. Fundova, D. Borovska, D. Srutkova, Z. Zidek, M. Schwarzer, P. Drastich and D. P. Funda, Cell Mol. Immunol., 2011, 8, 110–120 CrossRef CAS PubMed.
- N. Kamada, S.-U. Seo, G. Y. Chen and G. Nunez, Nat. Rev. Immunol., 2013, 13, 321–335 CrossRef CAS PubMed.
- F. Backhed, H. Ding, T. Wang, L. V. Hooper, G. Y. Koh, A. Nagy, C. F. Semenkovich and J. I. Gordon, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 15718–15723 CrossRef PubMed.
- C. A. Lozupone, J. I. Stombaugh, J. I. Gordon, J. K. Jansson and R. Knight, Nature, 2012, 489, 220–230 CrossRef CAS PubMed.
- H. Daniel, A. M. Gholami, D. Berry, C. Desmarchelier, H. Hahne, G. Loh, S. Mondot, P. Lepage, M. Rothballer, A. Walker, C. Bohm, M. Wenning, M. Wagner, M. Blaut, P. Schmitt-Kopplin, B. Kuster, D. Haller and T. Clavel, ISME J., 2014, 8, 295–308 CrossRef CAS PubMed.
- M. Kutschera, W. Engst, M. Blaut and A. Braune, J. Appl. Microbiol., 2011, 111, 165–175 CrossRef CAS PubMed.
- R. E. Ley, P. J. Turnbaugh, S. Klein and J. I. Gordon, Nature, 2006, 444, 1022–1023 CrossRef CAS PubMed.
- C. B. de La Serre, C. L. Ellis, J. Lee, A. L. Hartman, J. C. Rutledge and H. E. Raybould, Am. J. Physiol.: Gastrointest. Liver Physiol., 2010, 299, G440–G448 CrossRef CAS PubMed.
- D. Chen, S. B. Wan, H. Yang, J. Yuan, T. H. Chan and Q. P. Dou, Adv. Clin. Chem., 2011, 53, 155–177 CAS.
- L. Liu, L. Zubik, F. W. Collins, M. Marko and M. Meydani, Atherosclerosis, 2004, 175, 39–49 CrossRef CAS PubMed.
- W. Guo, E. Kong and M. Meydani, Nutr. Cancer, 2009, 61, 807–810 CrossRef CAS PubMed.
- B. Zou, C.-M. Li, J.-Y. Chen, X.-Q. Dong, Y. Zhang and J. Du, Food Res. Int., 2012, 48, 970–977 CrossRef CAS.
- U. Etxeberria, A. Fernández-Quintela, F. I. Milagro, L. Aguirre, J. A. Martínez and M. P. Portillo, J. Agric. Food Chem., 2013, 61, 9517–9533 CrossRef CAS PubMed.
- F. Cardona, C. Andrés-Lacueva, S. Tulipani, F. J. Tinahones and M. I. Queipo-Ortuño, J. Nutr. Biochem., 2013, 24, 1415–1422 CrossRef CAS PubMed.
- L. Valdes, A. Cuervo, N. Salazar, P. Ruas-Madiedo, M. Gueimonde and S. Gonzalez, Food Funct., 2015, 6, 2424–2439 CAS.
- X. Tzounis, A. Rodriguez-Mateos, J. Vulevic, G. R. Gibson, C. Kwik-Uribe and J. P. Spencer, Am. J. Clin. Nutr., 2011, 93, 62–72 CrossRef CAS PubMed.
- P. Dolara, C. Luceri, C. D. Filippo, A. P. Femia, L. Giovannelli, G. Caderni, C. Cecchini, S. Silvi, C. Orpianesi and A. Cresci, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2005, 591, 237–246 CrossRef CAS PubMed.
- J. Yamakoshi, S. Tokutake, M. Kikuchi, Y. Kubota, H. Konishi and T. Mitsuoka, Microb. Ecol. Health Dis., 2001, 13, 25–31 CrossRef CAS.
- A. Viveros, S. Chamorro, M. Pizarro, I. Arija, C. Centeno and A. Brenes, Poult. Sci., 2011, 90, 566–578 CrossRef CAS PubMed.
- M. Massot-Cladera, T. Pérez-Berezo, A. Franch, M. Castell and F. J. Pérez-Cano, Arch. Biochem. Biophys., 2012, 527, 105–112 CrossRef CAS PubMed.
- F. Cardona, C. Andres-Lacueva, S. Tulipani, F. J. Tinahones and M. I. Queipo-Ortuno, J. Nutr. Biochem., 2013, 24, 1415–1422 CrossRef CAS PubMed.
- T. Ozdal, D. A. Sela, J. Xiao, D. Boyacioglu, F. Chen and E. Capanoglu, Nutrients, 2016, 8, 78 CrossRef PubMed.
- H.-F. Gu, C.-M. Li, Y.-J. Xu, W.-F. Hu, M.-H. Chen and Q.-H. Wan, Food Res. Int., 2008, 41, 208–217 CrossRef CAS.
- S. Gahler, K. Otto and V. Böhm, J. Agric. Food Chem., 2003, 51, 7962–7968 CrossRef CAS PubMed.
- L. J. Porter, L. N. Hrstich and B. G. Chan, Phytochemistry, 1985, 25, 223–230 CrossRef.
- C. Li, R. Leverence, J. D. Trombley, S. Xu, J. Yang, Y. Tian, J. D. Reed and A. E. Hagerman, J. Agric. Food Chem., 2010, 58, 9033–9042 CrossRef CAS PubMed.
- J. Juśkiewicz and Z. Zduńczyk, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2002, 133, 411–417 CrossRef.
- F. A. Duca, Y. Sakar, P. Lepage, F. Devime, B. Langelier, J. Doré and M. Covasa, Diabetes, 2014, 63, 1624 CrossRef CAS PubMed.
- P. J. Turnbaugh, M. Hamady, T. Yatsunenko, B. L. Cantarel, A. Duncan, R. E. Ley, M. L. Sogin, W. J. Jones, B. A. Roe, J. P. Affourtit, M. Egholm, B. Henrissat, A. C. Heath, R. Knight and J. I. Gordon, Nature, 2009, 457, 480–484 CrossRef CAS PubMed.
- U. Etxeberria, N. Arias, N. Boqué, M. T. Macarulla, M. P. Portillo, J. A. Martínez and F. I. Milagro, J. Nutr. Biochem., 2015, 26, 651–660 CrossRef CAS PubMed.
- A. H. Smith, E. Zoetendal and R. I. Mackie, Microb. Ecol., 2005, 50, 197–205 CrossRef CAS PubMed.
- G. Galati and P. J. O'Brien, Free Radical Biol. Med., 2004, 37, 287–303 CrossRef CAS PubMed.
- R. Barczynska, K. Jochym, K. Slizewska, J. Kapusniak and Z. Libudzisz, J. Funct. Foods, 2010, 2, 126–133 CrossRef CAS.
- T. E. Cowan, M. S. Palmnas, J. Yang, M. R. Bomhof, K. L. Ardell, R. A. Reimer, H. J. Vogel and J. Shearer, J. Nutr. Biochem., 2014, 25, 489–495 CrossRef CAS PubMed.
- B. Zou, R. Nie, J. Zeng, Z. Ge, Z. Xu and C. Li, J. Funct. Foods, 2014, 11, 330–341 CrossRef CAS.
- B. Zou, Z. Ge, W. Zhu, Z. Xu and C. Li, Eur. J. Nutr., 2014, 54, 1333–1343 CrossRef PubMed.
- K. Matsumoto, S. Yokoyama and N. Gato, Phytother. Res., 2010, 24, 205–210 CAS.
- D. Del Rio, A. Rodriguez-Mateos, J. P. E. Spencer, M. Tognolini, G. Borges and A. Crozier, Antioxid. Redox Signaling, 2012, 18, 1818–1892 CrossRef PubMed.
- M. I. Queipo-Ortuño, M. Boto-Ordóñez, M. Murri, J. M. Gomez-Zumaquero, M. Clemente-Postigo, R. Estruch, F. Cardona Diaz, C. Andrés-Lacueva and F. J. Tinahones, Am. J. Clin. Nutr., 2012, 95, 1323–1334 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7fo00995j |
| This journal is © The Royal Society of Chemistry 2018 |