Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Low cost ionic liquid–water mixtures for effective extraction of carbohydrate and lipid from algae
Trang Q.
To
*a,
Kerryn
Procter
a,
Blake A.
Simmons
 b,
Suresh
Subashchandrabose
c and
Rob
Atkin
b,
Suresh
Subashchandrabose
c and
Rob
Atkin
 ad
ad
aPriority Research Centre for Advanced Fluids and Interfaces, Newcastle Institute for Energy and Resources, Australia. E-mail: Trang.To@newcastle.edu.au
bLawrence Berkeley National Laboratory, Joint BioEnergy Institute, Berkeley, CA 94720, USA
cGlobal Centre for Environmental Remediation, Faculty of Science, University of Newcastle, Callaghan, New South Wales 2308, Australia
dSchool of Molecular Sciences, The University of Western Australia, WA 6009, Australia
First published on 17th May 2017
Abstract
Biomass based biofuels are already an important energy source, and will increasingly be so in the future as the need for renewable energy rises. Due to their fast multiplication rates, algae can provide a sustainable supply of biomass, and are attractive because they do not compete with food crops for habitat. Here we show that biomass derived from Chlorella vulgaris and Spirulina platensis can be pretreated with low cost choline amino acid based ionic liquids to effectively yield lipids (30.6% and 51% total lipids) and sugars (71% and 26% total sugars). The ionic liquids dissolve the lipids, leaving behind a carbohydrate rich solid. The lipids were extracted with hexane, and the solid was subjected to enzyme hydrolysis to release fermentable sugars. These results open new pathways towards the dual production of biodiesel and bioethanol from algae, using low cost ionic liquids.
Introduction
As world fossil fuel resources begin to dwindle, alternative clean fuel production from renewable sources is not only preferred, but will also become compulsory in the future. In recent years, there has been increasing interest towards using biomass as a renewable energy source. Among the many types of biomass available, microalgae have gained attention for biodiesel production, due to their high lipid content.1–5 Microalgae are ubiquitous photosynthetic organisms that live in water and do not compete with food crops in terms of land and nutrient use. They harvest energy from the sun, CO2, and nutrients and minerals in the water environments in which they reside to synthesise a variety of complex macromolecules including carbohydrates, lipids and proteins. Microalgae can grow on wastewater that is rich in proteins and inorganic nitrogens, and so are used extensively in wastewater remediation;6,7 a short culture period of 14 days is sufficient for algae to remove up to 70–90% of nitrogen, phosphorus and COD (chemical oxygen demand) from winery and dairy wastewater.6 Microalgae are ideal platforms for biodiesel production due to their rapid multiplication rates and ability to accumulate a large mass of lipids that are readily converted through transesterification. Under suitable culture conditions, microalgae can accumulate up to 70% of lipids in dry biomass.8,9 Other than lipids, microalgae also contains significant amounts of carbohydrates (cellulose and starch), which can be converted to ethanol for further biofuel production. However, most studies on algae have focused solely on the lipids, with the carbohydrates neglected entirely.The most popular method to extract lipids from microalgae is Soxhlet extraction using hexane,10 but this approach has several disadvantages in terms of commercial viability. First, the cell walls of microalgae are made up of a highly complex matrix of polysaccharides intercalated with proteins,11–13 which has a high chemical resistance to non-polar solvents. Second, hexane is incapable of extracting lipids stored in lipid droplets, as it cannot cross the (protein bound) polar phospholipid-membrane. On the other hand, polar solvents such as methanol/chloroform cross the phospholipid barrier2 by diffusion and extract these lipids (the Bligh & Dyer method14). Physical pretreatment to break open the cells (such as osmotic shock,15 blending, microwave or laser16) followed by the extraction of lipids with solvents has been investigated, but these physical pretreatment steps, together with the need for dry algal biomass in the extraction, result in energy intensive processes that are not commercially feasible.2,3,17
Chemical pretreatment offers a potential alternative that may be commercially viable. For example, a biphasic system of acidic solution and hexane in a bioreactor (155 °C) facilitates the recovery of up to 97% of lipids and the release of 90% glucose from Chlorella and Scenedesmus.18 Certain ionic liquids (ILs), which consist entirely of cations and anions,19–21 are interesting candidates for the pretreatment step due to their favourable physical properties. ILs are tuneable, have the capacity to dissolve a wide range of materials, and low toxicity variants are known.20,21 ILs have attracted research interest for the pretreatment of lignocellulosic biomass for enhanced bioethanol production.22,23 Certain ILs selectively dissolve the lignin component of the lignocellulosic biomass, leaving behind a cellulose-rich residue easily digested by enzymes to release fermentable sugars.
The use of ILs for the pretreatment of algal biomass for lipid extraction has been investigated previously.24–29 ILs work by dissolving the lipids, which separate out of the liquor on addition of an antisolvent such as methanol, which are then recovered using hexane. However, improvement is needed in several areas.24 Most of the ILs employed in these studies are imidazolium- and pyridinium-based, which are ‘classic’ ILs but are expensive and toxic. Only two studies so far have used the cheaper ammonium- and phosphonium-based ILs.29,30 However, regardless of the cation type employed the algal biomass solid loading was low (between 5 and 10 wt%),31 and only a few studies have attempted to recover other valuable components of the algal biomass, such as carbohydrates and carotenoids.32–34
This study explores the use of a series of cheap and environmentally benign ILs for the pretreatment of microalgae. Made from simple acid–base reactions between two naturally occurring non-toxic chemicals, choline (an ammonium) and amino acids, these ILs are significantly cheaper and less toxic than imidazolium-based ILs. To further reduce the cost, instead of using neat ILs, mixtures of IL-water are utilised. This class of ILs has been shown to perform well for lignocellulosic biomass processing,35 and facilitate a one-pot reaction from biomass to ethanol. Two distinct, representative, microalgae are probed, one from the prokaryotic cyanobacterial group, Spirulina platensis, and the other from the eukaryotic green algae Chlorella vulgaris. The latter species C. vulgaris is extensively studied due to its high lipid content (∼300 mg g−1 dry cell). However, C. vulgaris possesses a robust and complex cell wall, which makes it resistant to chemical attack. In order to examine whether our ILs can penetrate this barrier, two samples of C. vulgaris were investigated, one with an intact cell wall, and one with a cracked cell wall. High solid loading (20 w/v%), moderate temperature (70 °C) and a short reaction time (3 h) were employed and both lipids and carbohydrates were recovered.
Materials and methods
Synthesis of ionic liquids
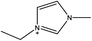
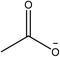
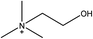
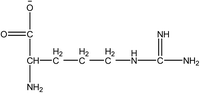
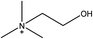
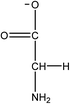
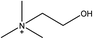
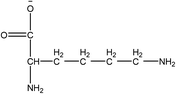
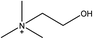
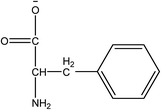
1-Ethyl-3-methylimidazolium acetate, choline hydroxide (46 wt% in water), all amino acids, namely L-arginine, glycine, L-lysine, and L-phenylalanine were purchased from Sigma Aldrich and used without any further purification. In the synthesis equal amounts (by mol%) of choline hydroxide and amino acids were mixed together. The resultant mixtures were heated up to 70 °C and stirred for 3 hours. Water was added to the mixture during heating to ensure that the amino acids had completely dissolved. Table 1 shows the structure of the ionic liquids, along with their water content; note that as natural biomass contains large volumes of water, the ILs were not dried prior to use.Pretreatment of algae with ILs
Biomass from two strains, Chlorella vulgaris (Algomed Germany, certified additive-free and untreated) and Spirulina platensis (Nourishme Organics) were sourced commercially. Food grade Chlorella vulgaris (cell wall broken by heating) was also obtained (Synergy Natural).0.5 g of the biomass samples was pretreated by dissolving in 2.5 mL of the selected ILs at 70 °C for 3 hours with continuous stirring. Ten mL of water was added and the samples were centrifuged to separate the solid and liquid fractions (6000 rpm, 15 minutes). The pretreated biomass solid was subjected to further washing with water (3 × 10 mL water) to remove residual IL.
Extraction of lipids from the IL liquors
The liquid fractions from centrifugation were acidified with concentrated hydrochloric acid (37 wt%). Acidification converts fatty acids to their neutral form that is more soluble in hexane. The acid was added dropwise until the solution reached pH 3. The lipids were extracted three times with hexane (3 × 5 mL), each time the centrifuge tubes were vigorously vortexed (15 seconds) followed by centrifugation (6000 rpm, 30 minutes). The hexane layers were collected and combined. Finally, hexane was removed under vacuum to yield crude lipid that was weighed using an analytical balance. The composition of the crude was analyzed to determine its purity and to calculate the actual lipid yield.Compositional analysis of the algal biomass and extracted crude lipids from the IL liquor
The original biomass and their solid fractions from centrifugation were analyzed to determine the total lipids, carbohydrate content, total solids and ash content. The crude lipids extracted from the IL liquors were analyzed for purity.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), HCl (0.6 M in methanol, 600 μL) and 2.645 mg of tridecanoic acid methyl ester that serves as the internal standard (C13:0ME, Sigma Aldrich, 25 μL of 10.58 mg mL−1 stock solution in hexane). The mixture was vortexed vigorously for 5 minutes before incubation at 85 °C for one hour, with brief vortexing every 15 minutes. The mixture was then left to cool to room temperature and 1 mL of hexane was added. The vial was vortexed again and left to stand for one hour. The hexane layer was extracted and sent for gas chromatography-mass spec (GC-MS) analysis.
1 v/v), HCl (0.6 M in methanol, 600 μL) and 2.645 mg of tridecanoic acid methyl ester that serves as the internal standard (C13:0ME, Sigma Aldrich, 25 μL of 10.58 mg mL−1 stock solution in hexane). The mixture was vortexed vigorously for 5 minutes before incubation at 85 °C for one hour, with brief vortexing every 15 minutes. The mixture was then left to cool to room temperature and 1 mL of hexane was added. The vial was vortexed again and left to stand for one hour. The hexane layer was extracted and sent for gas chromatography-mass spec (GC-MS) analysis.
The GC program (Shimadzu machine fitted with a Restek Rxi-5Sil MS column) was as follows: 1 μL injection at 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split ratio, inlet temperature of 250 °C; constant flow of 1 mL min−1 helium; oven temperature: 100 °C for 1 min, 25 °C min−1 up to 200 °C and hold for 1 min, 5 °C min−1 up to 250 °C and hold for 7 min (23 min total). For the first run, a full scan MS was carried out to identify the structure and the retention time of the FAMEs present. Selected ion monitoring (SIM) was then performed for enhanced sensitivity and accurate quantification. Calibration curves were created from standards made from a C8–C24 FAME mix (Supelco) and the C13:0ME internal standard. The Supelco mix covers most of the FAMEs present in the algal biomass, except for C16:2 and C16:3; whose quantification had to be done using the responsive factors obtained for C18:2n6 and C18:3n3 (present in the Supelco mix) as recommended in the NREL protocol.
1 split ratio, inlet temperature of 250 °C; constant flow of 1 mL min−1 helium; oven temperature: 100 °C for 1 min, 25 °C min−1 up to 200 °C and hold for 1 min, 5 °C min−1 up to 250 °C and hold for 7 min (23 min total). For the first run, a full scan MS was carried out to identify the structure and the retention time of the FAMEs present. Selected ion monitoring (SIM) was then performed for enhanced sensitivity and accurate quantification. Calibration curves were created from standards made from a C8–C24 FAME mix (Supelco) and the C13:0ME internal standard. The Supelco mix covers most of the FAMEs present in the algal biomass, except for C16:2 and C16:3; whose quantification had to be done using the responsive factors obtained for C18:2n6 and C18:3n3 (present in the Supelco mix) as recommended in the NREL protocol.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 30 seconds). The supernatant was collected and its carbohydrate concentration was measured using a ACCU-CHEK Performa Blood Glucose Meter (Roche Diagnostics).
000 rpm, 30 seconds). The supernatant was collected and its carbohydrate concentration was measured using a ACCU-CHEK Performa Blood Glucose Meter (Roche Diagnostics).
Saccharification by enzyme hydrolysis
An enzyme hydrolysis procedure was designed to target the cellulose and starch present in microalgae. The procedure was adopted from two standard protocols, the NREL protocol for lignocellulosic biomass38 and the Sigma Starch Assay protocol (STA-20).39In a GC vial, algal biomass (∼20 mg) was mixed together with 40 μg of tetracycline (4 μL of 10 mg mL−1 solution in EtOH), 30 μg of cycloheximide (3 μL of 10 mg mL−1 solution in water), 0.6 μL of cellulase (from Aspergillus sp., Sigma, equivalent to 25 U per g of biomass), 2 μL of α-amyloglucosidase (from Bacillus licheniformis, Sigma, equivalent to 25 U per g of biomass), 2 μL of α-amylase (from Aspergillus niger, Sigma, equivalent to 25 mg protein per g of biomass) and 970 μL of buffer solution (NaOAc/AcOH, pH 5). The vial was vortexed briefly then left at 50 °C with stirring for 24 h. The D-glucose and D-galactose content were measured using the Accu-check Performa Glucose meter.
Results and discussion
IL pretreatment of biomass was carried out with a high loading of 20 w/v% biomass/IL under very mild conditions (70 °C for 3 h). Five ionic liquids were tested in this study: four choline amino acid ILs together with a conventional IL, 1-ethyl-3-methylimidazolium acetate ([Emim][OAc]) (structures in Table 1). [Emim][OAc] is a benchmark IL for lignocellulosic biomass pretreatment.22,40 When [Emim][OAc] is used neat, cellulose is preferentially dissolved in the IL and then recovered in solid form using an antisolvent such as water or methanol. The recovered solid contains mostly cellulose with less lignin and hemicellulose than the starting material. This process is defined as the dissolution process.22 When [Emim][OAc] is used in a mixture with water (the Ionosolv process), lignin is preferentially dissolved, leaving behind a cellulose rich solid.40 The Ionosolv process reduces the amount of IL required. The choline amino ILs in this study were used as aqueous solutions (around 65% IL to 35% H2O, Table 1), i.e. following the Ionosolv concept, and are significantly cheaper in terms of starting materials and synthesis labour than [Emim][OAc]; synthesis is achieved by simply mixing choline hydroxide solution with an equivalent amount of amino acid to yield the IL within 3 hours without side products. The resultant IL water solutions were basic (pH 11–12.3).Moisture, solid, ash and TGA profiles
The main components of algae biomass are moisture, carbohydrates, proteins, lipids and ash. The TGA profiles of the three algae samples display similar composition, with 6–8% water, 85–89% solid and 3–7% ash (Table 2, Fig. 1–3). In addition to the primary mass loss profiles, the first derivative curves (dweight/dtemperature) were also analysed to better identify the mass loss sections. The first derivative curve of untreated C. vulgaris and S. platensis displayed three main peaks, corresponding to three stages of decomposition: dehydration (50–200 °C), devolatization of the hydrocarbon chains of the lipids, cellulose, hemicellulose, starch and proteins (200–500 °C), and decomposition of the carbonaceous solids (500–600 °C). These finding are similar to the reported TGA profiles of green algae.41Fig. 1–3 show that after pretreatment with ILs the third decomposition stage shifts to a lower temperature, signifying fragmentation of the biomass to lower molecular weight molecules. The largest shift in all three algae samples can be seen for [Ch][ARG] (Fig. 1–3, blue line), signifying that this IL was better than all the other ILs in digesting the algal biomass. This compliments the solid recovery results (Table 2), in which [Ch][ARG] pretreatment resulted in the lowest solid recovery (30.4% for intact C. vulgaris, 25.0% for broken C. vulgaris and 24.1% for S. platensis).| TGA results | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorella vulgaris (Algomed, intact) | Chlorella vulgaris (Synergy, cell broken) | Spirulina platensis | |||||||||||||||
| Raw | [Emim][OAc] | [Ch][ARG] | [Ch][GLY] | [Ch][LYS] | [Ch][PHE] | Raw | [Emim][OAc] | [Ch][ARG] | [Ch][GLY] | [Ch][LYS] | [Ch][PHE] | Raw | [Emim][OAc] | [Ch][ARG] | [Ch][GLY] | [Ch][LYS] | |
| Moisture% | 7.2 | 10.0 | 14.9 | 10.1 | 11.8 | 10.1 | 7.9 | 6.0 | 8.1 | 8.0 | 7.6 | 7.6 | 6.2 | 7.2 | 7.8 | 7.8 | 8.9 |
| Solid% | 85.7 | 84.6 | 85.1 | 85.3 | 87.9 | 84.7 | 88.4 | 92.7 | 88.8 | 92.0 | 92.0 | 92.4 | 88.7 | 89.6 | 90.2 | 89.5 | 91.1 |
| Ash% | 7.1 | 5.4 | 0 | 4.6 | 0.3 | 5.2 | 3.7 | 1.3 | 3.1 | 0 | 0.4 | 0 | 5.1 | 3.2 | 2.0 | 2.7 | 0 |
| Solid recovery (%) | 67.4 | 30.4 | 74.5 | 59.6 | 66.9 | 52.9 | 25.0 | 61.3 | 68.4 | 61.0 | 82.3 | 24.1 | 51.8 | 38.8 | |||
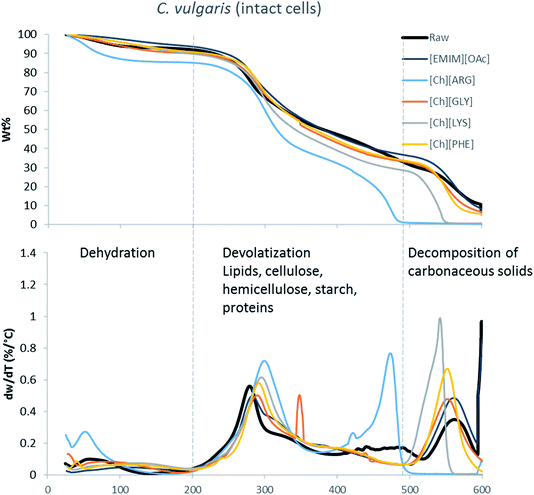 | ||
| Fig. 1 TGA profiles and their first derivative curves for intact C. vulgaris and its treated counterparts. | ||
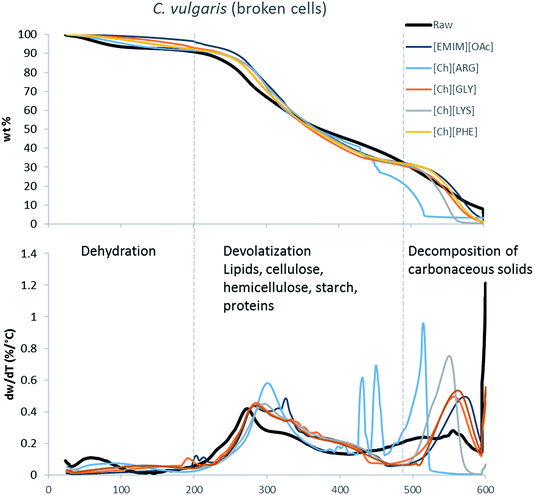 | ||
| Fig. 2 TGA profiles and their first derivative curves for broken C. vulgaris and its treated counterparts. | ||
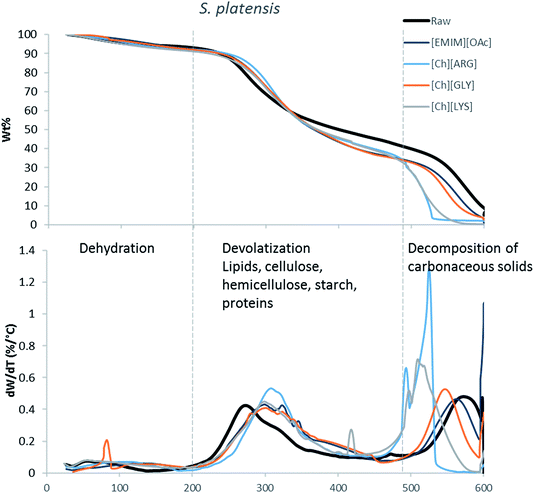 | ||
| Fig. 3 TGA profiles and their first derivative curves for S. platensis and its treated counterparts. | ||
There is a consistent reduction of ash in all of the pretreated biomass (see Table 2) samples. Ash is composed of minerals together with silica, which can be dissolved using alkali solution. Hence it is not surprising that some of the ash was lost during the pretreatment, since the IL solutions were all basic.
Effect of the pretreatment on algal lipids
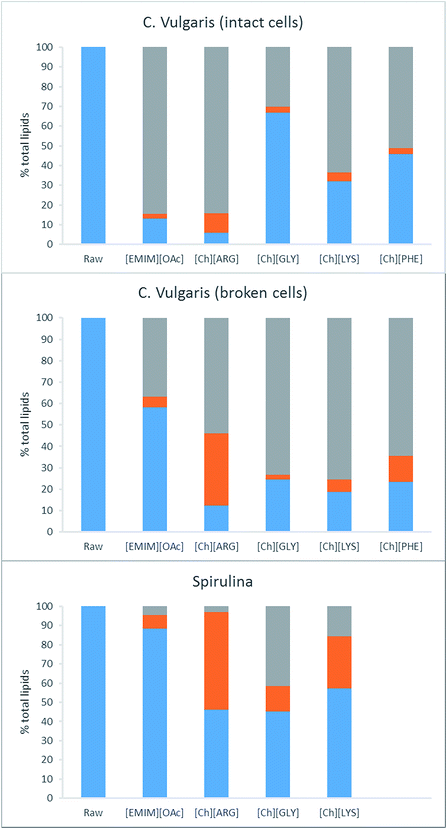
The two Chlorella vulgaris samples had similar lipid contents (287.9 and 302.8 mg g−1 total lipids/dry solid) while the S. platensis strain only had 102.7 mg g−1 (see Table 3). C18 long fatty acids constitute the majority of the lipids in all three samples. Pretreatment of the algae with ILs resulted in a partial dissolution of the lipids into the IL solutions. The lipid content of the solid residues ranged widely from 5.8% to 88.3% of the starting values. [Ch][ARG] was the most effective IL, leaving behind only 5.8% (intact C. vulgaris), 12.5% (broken C. vulgaris) and 46.3% (S. platensis) of the starting lipids. In comparison, [Emim][OAc] was less efficient. It dissolved lipids for the intact C. vulgaris (13.1% lipids remaining in the solid), but not as effectively for the broken forms of C. vulgaris (58.3%) and the S. platensis (88.3%). The other three choline-amino acid ILs behaved similarly to each other, working well for the broken form of C. vulgaris (18.8–24.5%), and moderately for the intact C. vulgaris (32.0–67.0%) and S. platensis (45.2 and 57.3%) (note: S. platensis was not tested with [Ch][PHE] because a preliminary experiment showed poor performance). It is worth noting that [Ch][ARG] and [Emim][OAc] dissolved more lipids from the intact C. vulgaris than the broken form, which is unusual since the intact C. vulgaris possesses a strong cell wall that should inhibits dissolution. Disparity in the sample sources might account for this abnormality; or the process of cell wall breaking by heating could have destroyed some lipids that if left intact could have been solubilized by the ILs studied. Regardless of the sample type, it is clear that [Ch][ARG] was superior over other ILs in removing lipids from the samples of C. vulgaris.| Fatty acids | Chlorella vulgaris (Algomed, intact) | Chlorella vulgaris (Synergy, cell broken) | Spirulina platensis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | [Emim][OAc] | [Ch][ARG] | [Ch][GLY] | [Ch][LYS] | [Ch][PHE] | Raw | [Emim][OAc] | [Ch][ARG] | [Ch][GLY] | [Ch][LYS] | [Ch][PHE] | Raw | [Emim][OAc] | [Ch][ARG] | [Ch][GLY] | [Ch][LYS] | |
| Fatty acid composition of solid residue (% of total lipids) | |||||||||||||||||
| C16:0 | 17.3 | 10.2 | 25.2 | 17.4 | 22.5 | 22.3 | 13.2 | 16.1 | 45.0 | 26.7 | 18.6 | 25.1 | 34.1 | 36.6 | 64.3 | 38.3 | 48.2 |
| C16:1 | 3.7 | 4.6 | ND | 4.1 | 5.2 | 6.6 | 2.1 | 3.6 | ND | 2.4 | 4.9 | 2.9 | 8.2 | 9.3 | 0.9 | 5.6 | 4.1 |
| C16:2 | 4.8 | 1.3 | ND | 3.1 | 1.6 | 5.4 | 5.3 | 6.2 | ND | 1.8 | 1.0 | 3.5 | ND | ND | ND | ND | ND |
| C16:3 | 10.5 | 5.4 | ND | 5.3 | 1.8 | 9.6 | 4.5 | 4.7 | ND | ND | ND | 1.0 | 0.4 | ND | ND | ND | ND |
| C18:0 | 1.4 | ND | ND | 2.2 | 2.1 | 1.1 | 2.9 | 3.3 | 4.7 | 4.2 | 1.4 | 3.7 | 0.1 | 0.5 | 0.4 | 0.6 | 1.5 |
| C18:1 | 10.3 | 2.9 | ND | 13.2 | 13.3 | 9.4 | 8.8 | 8.3 | 1.2 | 10.0 | 5.0 | 8.2 | ND | 1.2 | ND | 0.7 | 2.7 |
| C18:2 | 19.7 | 10.8 | 0.7 | 22.6 | 22.1 | 17.8 | 33.1 | 32.5 | 17.8 | 32.2 | 28.6 | 29.9 | 17.0 | 17.2 | 7.3 | 17.5 | 18.9 |
| C18:3 | 28.2 | 11.1 | ND | 25.8 | 21.2 | 22.0 | 25.1 | 22.4 | 1.3 | 10.0 | 9.0 | 11.9 | 21.7 | 18.7 | ND | 10.3 | 7.6 |
| C22 | 2.1 | 26.5 | 36.4 | 3.1 | 5.0 | 2.9 | 2.7 | 1.6 | 14.8 | 6.3 | 15.5 | 6.9 | 9.1 | 8.0 | 13.3 | 13.3 | 8.3 |
| C22:1 | 2.1 | 27.4 | 37.6 | 3.2 | 5.1 | 3.0 | 2.4 | 1.3 | 15.1 | 6.3 | 16.0 | 6.9 | 9.4 | 8.3 | 13.8 | 13.8 | 8.7 |
| Total | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| mg g−1 dry solid | 287.9 | 56.1 | 55.4 | 258.7 | 154.7 | 197.3 | 300.1 | 331.1 | 149.6 | 119.8 | 82.4 | 115.6 | 102.7 | 110.1 | 197.2 | 89.5 | 151.4 |
| % total lipids | 13.1 | 5.8 | 67.0 | 32.0 | 45.8 | 58.3 | 12.5 | 24.5 | 18.8 | 23.5 | 88.3 | 46.3 | 45.2 | 57.3 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||||||||
| Lipid extracted from the IL liquor by hexane | |||||||||||||||||
| mg g−1 original biomass | 7.1 | 28.8 | 8.0 | 13.3 | 8.7 | 14.7 | 100.7 | 6.8 | 17.3 | 36.0 | 7.4 | 52.5 | 13.7 | 28.0 | |||
| % total lipids | 2.5 | 10.0 | 2.8 | 4.6 | 3.0 | 4.9 | 33.6 | 2.3 | 5.8 | 12.0 | 7.2 | 51.1 | 13.3 | 27.3 | |||
The second step of retrieving the lipids involves extracting them from the IL liquor using hexane. The IL liquors were neutralized with acid, and the lipids were extracted using hexane. Without neutralization, no fatty acids were recovered from the IL liquors. Neutralization allows the fatty acids to convert to their neutral form, which is more soluble in hexane than the anionic form. The purity of the crude lipid extracts was analyzed using GC and was found to vary between 20 and 100%. These values were taken into account to calculate the actual lipid yield. In cells, lipids are stored as triacylglycerols in free form and also in lipid droplets, surrounded by a phospholipid monolayer decorated with proteins. For successful extraction of lipids, pretreatment must break down the cell wall and the cell membrane, as well as release the lipids from any bound proteins. It was found that not all the lipids that were removed from the biomass could be extracted using the hexane. Fig. 4 displays the distribution of lipids after pretreatment. [Ch][ARG] further displays its superiority over the other ILs in disintegrating lipids, as the hexane was able to extract 10.0% (intact C. vulgaris), 33.6% (broken C. vulgaris) and 51.1% (S. platensis) of the total lipids (Table 1 and Fig. 4, orange bars). It is also evident that the broken form of C. vulgaris yielded more lipid than the intact form (33.6% compared to 10.0%), despite initially dissolving less into the IL liquor. This implies that the process of cell wall breaking does help downstream lipid extraction, allowing the IL to penetrate the cells and disintegrate lipids better. The pros and cons of the cell wall breaking pretreatment are discussed in the last section.
For the other ILs, the majority of the dissolved lipids stayed in the liquor, possibly due to the lipids still being bound within the lipid droplets, or bound to proteins, unextractable using hexane. Hexane was able to extract 2.5%, 4.5% and 7.2 wt% (intact C. vulgaris, broken C. vulgaris and S. platensis) of the total lipids out of the [Emim][OAc] liquors. These values are lower than the reported values for [Emim][OAc] pretreatment with C. vulgaris using other methods.24,25,28 It is worth noting that the extraction time was short (vigorous shaking followed by immediate centrifugation to separate the hexane layer), unlike other methods in which the IL/biomass mixtures were stirred with hexane for hours.25 Intact C. vulgaris proved difficult for all but [Ch][ARG], extracting yield for [Emim][OAc] and the other three amino acid ILs were all less than 5%. Significant yields were noted for [Ch][LYS]/S. platensis (27.1%) and [Ch][PHE]/broken C. vulgaris (12.0% yield).
One possible explanation for the effectiveness of [Ch][ARG] is the structure of the anion, as compared to the other IL anions. Studies have found that in applications involving ILs, the anion plays an important role in dissolving substrates, much more so than the cation.22 In lignocellulosic biomass pretreatment, the ILs that worked well all contained good H-bond acceptor anions e.g. Cl− or OAc−,22,40,42 that allow the ILs to bind to –OH groups that are present in the cellulose and proteins in the biomass. All amino acid anions have at least 3 hydrogen bonding sites, one from the amino NH2 and two from the carboxylate COO−. In this study, the argininate anion has three extra nitrogens on its branch, allowing a total of 6 hydrogen bonding sites to bind to the biomass. [Ch][LYS] also has one extra nitrogen on the anion, which might explain its better performance than [Ch][GLY]. [Ch][PHE] does not have any extra hydrogen bonding sites, but does have an aromatic phenyl ring, which suggests that π–π stacking might be playing a role in the interaction between [Ch][PHE] and the C. vulgaris cells.
Effect of the pretreatment on carbohydrates
Carbohydrate composition of the raw biomass and their pretreated counterparts was determined using a two-step acid hydrolysis according to the NREL protocol. Previous studies on the carbohydrate content of algal biomass showed that the two most abundant monomeric sugars present in C. vulgaris are D-galactose and D-glucose (49 wt% and 35 wt% of total carbohydrate respectively).43 The ACCU-CHEK Performa glucose meter used was responsive to both D-glucose and D-galactose. This is not unusual, for several glucose-meters have been reported to give unusually high readings in the presence of other sugars such as maltose or galactose.44 While this is not desirable in a clinical setting, it conveniently provided us with a quick way to measure multiple sugars simultaneously. Our calibration indicated that this meter is responsive almost equally to glucose and galactose, and measurement of 5 different mixtures of glucose and galactose produced readings that were all within 10% error of the total sugar content. Hence readings are efficiently the sum of D-glucose and D-galactose, which conveniently constitutes 84 wt% of the total carbohydrate present in the algal biomass.C. vulgaris contained 296.4 mg carbohydrate per g of biomass (intact form), 133.4 mg g−1 (broken form) while S. platensis contained only 85.2 mg g−1 (Table 4). S. platensis is consumed as a dietary fibre supplement, credited to its fibrous and low calorie nature, in line with its lows lipid (102.7 mg g−1) and low sugar compositions. Pretreatment of C. vulgaris (both samples) produced solids richer in carbohydrates. Most exceptional was [Ch][ARG] which produced 800.7 mg g−1carbohydrate rich solid (equivalent to 82% starting sugars when solid recovery of 30.4% is taken into account). Combined with the lipid value, it is evident that this IL had carried out a highly precise fractionation of the biomass, for it has selectively dissolved the lipids of the biomass to produce a sugar rich solid. The effect was much more pronounced for the intact form of C. vulgaris than the broken form (362.8 mg g−1 sugar per dry solid residue), likely due to the carbohydrate storage (mostly as starch) in the broken cells being partially destroyed in the process of cell-wall breaking, consequently allowing free sugars to dissolve into the aqueous solution of the IL liquors. Other ILs produced solids less rich in carbohydrates than [Ch][ARG], but still retained 85–103% starting sugars from the intact C. vulgaris, and 52–79% from the broken C. vulgaris. It is clear that more sugar was lost from the broken C. vulgaris than the intact form. By contrast, in the case of S. platensis, only pretreatment with [Emim][OAc] preserves the sugar in the solid (94% sugar retained), choline-amino acid pretreatment resulted in a large loss of sugars. S. platensis has a softer cell wall than C. vulgaris, which must have allowed the sugars to dissolve into the aqueous solution of the choline amino acid ILs.
| Carbohydrate (D-glucose + D-galactose) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorella vulgaris (Algomed, intact) | Chlorella vulgaris (Synergy, cell broken) | Spirulina platensis | |||||||||||||||
| Raw | [Emim][OAc] | [Ch][ARG] | [Ch][GLY] | [Ch][LYS] | [Ch][PHE] | Raw | [Emim][OAc] | [Ch][ARG] | [Ch][GLY] | [Ch][LYS] | [Ch][PHE] | Raw | [Emim][OAc] | [Ch][ARG] | [Ch][GLY] | [Ch][LYS] | |
| mg g−1 dry solid | 296.4 | 372.8 | 800.7 | 378.0 | 475.5 | 455.7 | 133.4 | 124.5 | 362.8 | 144.4 | 141.6 | 148.4 | 85.2 | 97.2 | 99.0 | 79.5 | 42.5 |
| mg g−1 original biomass | 296.4 | 251.2 (85%) | 243.4 (82%) | 281.6 (95%) | 283.4 (96%) | 304.7 (103%) | 133.4 | 70.0 (52%) | 104.0 (78%) | 96.3 (72%) | 104.9 (79%) | 98.0 (73%) | 85.2 | 80.0 (94%) | 23.8 (28%) | 41.2 (48%) | 16.5 (19%) |
| Enzyme mg g−1 original biomass | 97.0 (33%) | 133.5 (45%) | 210.0 (71%) | 194.0 (65%) | 173.7 (59%) | 162.3 (55%) | 28.8 (22%) | 13.5 (10%) | 31.3 (23%) | 29.5 (22%) | 24.2 (18%) | 28.2 (21%) | 19.8 (23%) | 11.9 (14%) | 22.2 (26%) | 15.3 (18%) | 13.9 (16%) |
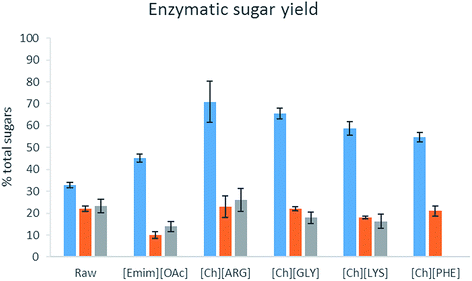
The bioethanol production potential of raw biomass and their pretreated counterparts was estimated using enzymatic hydrolysis. A concoction of α-amylase, α-amyloglucosidase and cellulase was used to digest the biomass (as carbohydrates are present in both cellulose and starch). The amount ofD-glucose and D-galactose released per g of the original biomass (i.e. loss of solid during the pretreatment step was taken into account) is reported in Table 4 and Fig. 5. Unlike lignocellulosic biomass, which usually has a very high resistance to enzymes, the raw algal biomass did produce sugar upon enzyme hydrolysis, albeit not completely. Pretreatment was beneficial to the intact form of C. vulgaris, for all the IL pretreatment helped produce more sugar. [Ch][ARG] and [Ch][GLY] were exceptional and helped produce more than double the amount of sugars (released 71% and 65% of total sugars) as compared to the untreated sample (33%). On the other hand, for the broken form of C. vulgaris and S. platensis, there was not much improvement. This could be explained by the loss of sugar to the aqueous solution of the ILs. However, it is not clear why [Emim][OAc] pretreated S. platensis showed such a low activity towards enzyme hydrolysis, despite being richer in carbohydrate. [Emim][OAc] might have carried out inadequate fractionation, in which only the outer parts of the algae were removed while the core cell structure was still intact and resistant to enzymes; or there might have been strong attachment of the [Emim][OAc] molecules to the algal biomass which consequently poisoned and diminished the enzyme activity.
Factors to consider for commercialization – comparing the behaviour of intact and broken C. vulgaris
Model studies by Haas et al. had shown that dewatering and drying of biomass constitutes 70% of the energy required for biodiesel production from algal biomass.45 As the choline-amino acid ILs used in this work are aqueous solutions this means dried algae is not required. Furthermore, simple synthesis and cheap starting materials are certainly advantageous in terms of commercial viability. Recycling of the ILs is likely possible (though not investigated yet in this paper). We envisage addition of an alkali (e.g. NaOH) to neutralize the added acid followed by the removal of excess water will effect recycling of the IL. Accumulation of biomass residuals in the ILs might occur after repeated cycles and its consequence will need to be examined.The question as to whether physical pretreatment is required remains open. As [Ch][ARG] produced the best results both in terms of lipids and sugar yields, it will be used to discuss the pros and cons of physical pretreatment. Cell disruption techniques for C. vulgaris (e.g. using pressure, ultrasonication, ozonation46 or enzymes47) certainly demand energy. Intact C. vulgaris fractionated better than broken ones, for 5.8% of total lipids and 82% of total sugars remained in the solid, cf. 12.5% and 78% for broken C. vulgaris. Enzymatic hydrolysis results were also better for the pretreated intact C. vulgaris (71% of total sugars was released compared to only 23% from the broken cells). However, the dissolved lipids of intact C. vulgaris were not easily recovered using hexane (only 10.0% was extracted, compared to 33.6% from the broken cells). Nevertheless, optimization of the extracting technique (e.g. longer immersion time with hexane, or addition of methanol as an anti-solvent instead of acid) might help improve the lipid yield. Taking everything into account, our opinion is that using intact cells and skipping any physical treatment offers a more economical solution. Optimization of the extraction step has to be investigated in future work.
Conclusion
We have demonstrated the use of a series of low cost ILs and [Emim][OAc] for the pretreatment of algal biomass. Among the highlights are the simple synthesis of the choline amino acids, the use of aqueous solutions (65% IL to 35% water) instead of neat ILs, mild pretreatment conditions (70°C, 3 h), high loading (20 w/v%) and extraction of up to 51.1% of total lipids. Most exceptional was choline argininate, which yielded the most lipids and sugars. The structure of the argininate anion is likely responsible for its effectiveness, for it contains 6 hydrogen-bonding sites to interact with the biomass. Along with lipids, ash was also removed during the pretreatment, due to the basicity of the IL solutions dissolving the silica in the ash. Our results show physical pretreatment of C. vulgaris, to break open the cell wall prior to chemical pretreatment, is not necessary. While physical pretreatment does yield more lipids, the additional energy demand, reduction in fractionation efficiency and lower sugar yield are unfavourable. Simple optimization of the extraction step (such as longer soaking time with hexane or using MeOH as an antisolvent) might help maximize the yield of lipid when the intact C. vulgaris are used. Recycling of the ILs and optimization of the extraction step would be the subject of further study.Acknowledgements
The authors acknowledge the financial support provided by the Priority Research Centre for Advanced Fluids and Interfaces, the University of Newcastle, Australia. Rob Atkin thanks the Australian Research Council for a Future Fellowship. The work by the DOE Joint BioEnergy Institute (http://www.jbei.org/) is supported by the DOE Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the DOE.References
- A. Singh, P. S. Nigam and J. D. Murphy, Bioresour. Technol., 2011, 102, 10–16 CrossRef CAS PubMed.
- R. Halim, M. K. Danquah and P. A. Webley, Biotechnol. Adv., 2012, 30, 709–732 CrossRef CAS PubMed.
- M. K. Lam and K. T. Lee, Biotechnol. Adv., 2012, 30, 673–690 CrossRef CAS PubMed.
- R. R. Kumar, P. H. Rao and M. Arumugam, Front. Energy Res., 2015, 2, 1–9 Search PubMed.
- J. J. Milledge, Rev. Environ. Sci. Biotechnol., 2011, 10, 31–41 CrossRef.
- C. X. Liu, S. Subashchandrabose, H. Ming, B. Xiao, R. Naidu and M. Megharaj, J. Appl. Phycol., 2016, 28, 3331–3341 CrossRef CAS.
- C. X. Liu, S. R. Subashchandrabose, M. Megharaj, Z. Q. Hu and B. Xiao, Bioresour. Technol., 2016, 218, 1170–1177 CrossRef CAS PubMed.
- C. H. Hsieh and W. T. Wu, Bioresour. Technol., 2009, 100, 3921–3926 CrossRef CAS PubMed.
- G. Van Vooren, F. Le Grand, J. Legrand, S. Cuine, G. Peltier and J. Pruvost, Bioresour. Technol., 2012, 124, 421–432 CrossRef CAS PubMed.
- A. L. Ahmad, N. H. M. Yasin, C. J. C. Derek and J. K. Lim, Renewable Sustainable Energy Rev., 2011, 15, 584–593 CrossRef CAS.
- D. S. Domozych, M. Ciancia, J. U. Fangel, M. D. Mikkelsen, P. Ulvskov and W. G. T. Willats, Front. Plant Sci., 2012, 3, 82 CAS.
- A. M. Aboshady, Y. A. Mohamed and T. Lasheen, Biol. Plant., 1993, 35, 629–632 CrossRef CAS.
- E. Kapaun, E. Loos and W. Reisser, Phytochemistry, 1992, 31, 3103–3104 CrossRef CAS.
- E. G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef CAS PubMed.
- A. R. Byreddy, A. Gupta, C. J. Barrow and M. Puri, Mar. Drugs, 2015, 13, 5111–5127 CrossRef CAS PubMed.
- J. R. McMillan, I. A. Watson, M. Ali and W. Jaafar, Appl. Energy, 2013, 103, 128–134 CrossRef.
- H. C. Greenwell, L. M. Laurens, R. J. Shields, R. W. Lovitt and K. J. Flynn, J. R. Soc., Interface, 2010, 7, 703–726 CrossRef CAS PubMed.
- L. M. L. Laurens, N. Nagle, R. Davis, N. Sweeney, S. Van Wychen, A. Lowell and P. T. Pienkos, Green Chem., 2015, 17, 1145–1158 RSC.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1–56 CrossRef CAS.
- A. Brandt, J. Grasvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- A. A. Elgharbawy, M. Z. Alam, M. Moniruzzaman and M. Goto, Biochem. Eng. J., 2016, 109, 252–267 CrossRef CAS.
- V. C. A. Orr and L. Rehmann, Current Opinion in Green and Sustainable Chemistry, 2016, 2, 22–27 CrossRef.
- S.-A. Choi, J.-S. Lee, Y.-K. Oh, M.-J. Jeong, S. W. Kim and J.-Y. Park, Algal Res., 2014, 3, 44–48 CrossRef.
- Y. H. Kim, S. Park, M. H. Kim, Y. K. Choi, Y. H. Yang, H. J. Kim, H. Kim, H. S. Kim, K. G. Song and S. H. Lee, Biomass Bioenergy, 2013, 56, 99–103 CrossRef CAS.
- Y. H. Kim, Y. K. Choi, J. Park, S. Lee, Y. H. Yang, H. J. Kim, T. J. Park, Y. H. Kim and S. H. Lee, Bioresour. Technol., 2012, 109, 312–315 CrossRef CAS PubMed.
- S. A. Choi, Y. K. Oh, M. J. Jeong, S. W. Kim, J. S. Lee and J. Y. Park, Renewable Energy, 2014, 65, 169–174 CrossRef CAS.
- M. Olkiewicz, M. P. Caporgno, J. Font, J. Legrand, O. Lepine, N. V. Plechkova, J. Pruvost, K. R. Seddon and C. Bengoa, Green Chem., 2015, 17, 2813–2824 RSC.
- V. C. A. Orr, N. V. Plechkova, K. R. Seddon and L. Rehmann, ACS Sustainable Chem. Eng., 2016, 4, 591–600 CrossRef CAS.
- J. M. Restrepo-Florez, A. Bassi, L. Rehmann and M. R. Thompson, Bioresour. Technol., 2013, 147, 456–463 CrossRef CAS PubMed.
- K. Gao, V. Orr and L. Rehmann, Bioresour. Technol., 2016, 206, 77–85 CrossRef CAS PubMed.
- L. B. Malihan, G. M. Nisola, N. Mittal, J. G. Seo and W. J. Chung, Renewable Energy, 2014, 66, 596–604 CrossRef CAS.
- T. J. Trivedi and A. Kumar, Green Sustainable Chem., 2014, 4, 190–201 CrossRef.
- M. J. Liszka, A. Kang, N. V. S. N. M. Konda, K. Tran, J. M. Gladden, S. Singh, J. D. Keasling, C. D. Scown, T. S. Lee, B. A. Simmons and K. L. Sale, Green Chem., 2016, 18, 4012–4021 RSC.
- S. V. Wychen, K. Ramirez and L. M. L. Laurens, Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by in situ Transesterification, National Renewable Energy Laboratory, 2013 Search PubMed.
- S. V. Wychen and L. M. L. Laurens, Determination of total carbohydrates in algal biomass, National Renewable Energy Laboratory, 2015 Search PubMed.
- M. Selig, N. Weiss and Y. Ji, Enzymatic Saccharification of Lignocellulosic Biomass, Report NREL/TP-51042629, National Renewable Energy Laboratory, 2008 Search PubMed.
- STA-20 Starch Assay Kit (Amylase/Amyloglucosidase Method), Sigma.
- A. Brandt, M. J. Ray, T. Q. To, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2011, 13, 2489–2499 RSC.
- K. Kebelmann, A. Hornung, U. Karsten and G. Griffiths, Biomass Bioenergy, 2013, 49, 38–48 CrossRef CAS.
- K. Dong, S. J. Zhang and J. J. Wang, Chem. Commun., 2016, 52, 6744–6764 RSC.
- D. W. Templeton, M. Quinn, S. Van Wychen, D. Hyman and L. M. L. Laurens, J. Chromatogr. A, 2012, 1270, 225–234 CrossRef CAS PubMed.
- T. G. Schleis, Pharmacotherapy, 2007, 27, 1313–1321 CrossRef CAS PubMed.
- M. J. Haas, A. J. McAloon, W. C. Yee and T. A. Foglia, Bioresour. Technol., 2006, 97, 671–678 CrossRef CAS PubMed.
- Y. Huang, A. Hong, D. Zhang and L. Li, Environ. Technol., 2014, 35, 931–937 CrossRef CAS PubMed.
- H. G. Gerken, B. Donohoe and E. P. Knoshaug, Planta, 2013, 237, 239–253 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |