Open Access Article
Open Access ArticleCellulose-based water purification using paper filters modified with polyelectrolyte multilayers to remove bacteria from water through electrostatic interactions
Anna
Ottenhall
 *,
Jonatan
Henschen
*,
Jonatan
Henschen
 ,
Josefin
Illergård
,
Josefin
Illergård
 and
Monica
Ek
and
Monica
Ek
 *
*
Department of Fibre and Polymer Technology, KTH Royal Institute of Technology, Teknikringen 56-58, 114 28 Stockholm, Sweden. E-mail: aott@kth.se; hens@kth.se; josefika@kth.se; monicaek@kth.se
First published on 3rd October 2018
Abstract
Filtration is a common way to obtain pure drinking water by removing particles and microorganisms based on size exclusion. Cellulose-based filters are affordable and biobased option for the removal of particles but bacteria are usually too small to be removed by size exclusion alone. In this article, the surfaces of cellulose fibres in two types of commercial paper filters have been given a positive net charge to trap bacteria through electrostatic interactions without releasing any biocides. The fibres were modified with the cationic polyelectrolyte polyvinylamine polymer in single layers (1 L) or in multilayers together with the anionic polyelectrolyte polyacrylic acid (3 L or 5 L) using a water-based process at room temperature. Filtration tests show that all filters, using both types of filter papers and a number of layers, can physically remove more than 99.9% of E. coli from water and that the 3 L modified filters can remove more than 97% of cultivatable bacteria from natural water samples. The bacterial reduction increased with increasing number of filter sheets used for the filtration and the majority of the bacteria were trapped in the top sheets of the filter. The results show the potential for creating water purification filters from bio-based everyday consumable products with a simple modification process. The filters could be used in the future for point-of-use water purification that may be able to save lives without releasing bactericides.
Water impactPaper filters were given a positive surface charge, through LbL adsorption, to trap bacteria through electrostatic interactions while keeping large pores in the filters. This method for making bio-based filters could be a sustainable alternative for disposable point-of-use water purification in the future, using an affordable and environmentally friendly production process while reducing plastic waste in the environment. |
1. Introduction
Cheap and simple decontamination of water could save millions of lives around the world as drinking water contaminated with faecal bacteria causes life-threatening diseases such as cholera and other water-borne diseases.1–3 Although many areas in developing countries need better access to large-scale water purification plants, it is also important to provide portable point-of-use (POU) methods for instant water purification in e.g. rural areas that lack infrastructure.4–6The interest in the use of biobased filters for water purification has increased in recent years, as such filters have the potential to be affordable, lightweight and biodegradable.7 Wood-based cellulose pulp fibres are today used in disposable everyday filters, such as coffee filters and air filters, and some of the first membranes for microfiltration were produced from cellulose nitrate and cellophane in the late 1920s.8 Microfiltration uses membranes with a pore size less than 0.5 μm to physically remove bacteria from water, such as faecal bacteria e.g. Escherichia coli and Vibrio cholera, which are approximately 1–2 μm in length.9,10 Lately, research has been focused on creating biobased membranes for micro- and ultrafiltration from cellulose nanofibrils (CNFs).11,12 For example, Ma et al. have used electrospun ultrafine nanofibers made from cellulose to create a barrier layer on petroleum-based membranes to achieve a pore size of approximately 20 nm and Mautner et al. have used CNF paper with a pore size of a few nanometers as a membrane for ultrafiltration.13,14 The drawbacks of membrane filtration are that the small pores impair the water flow, so that electrical pumps are often required to obtain a sufficient flow through the filter, and that the small pores in the membranes can easily become clogged by organic matter in water.9,15
An option for simple biobased POU water filtration is to use paper filters made of cellulose fibres. Filters based on cellulose pulp fibres do usually have large pores that facilitate water percolation but they do not sufficiently remove bacteria through size exclusion; other techniques are therefore needed to achieve a bacteria-reducing effect. Several groups have addressed this issue by incorporating antibacterial metal nanoparticles into cellulose-based water filters; both silver nanoparticles (AgNPs) and copper nanoparticles (CuNPs) are known to have good antibacterial effects.4,16,17 Dankovich et al. have impregnated paper with AgNPs and CuNPs, which inactivated bacteria during percolation through the filter and a 5 log10 reduction was reported for highly contaminated waters from streams in the Limpopo Province, South Africa.4,18,19 These metal nanoparticles can however have a negative impact on the environment if they end up in water sources as both AgNPs and CuNPs are acutely toxic towards aqueous organisms.20–25 Additionally, the exposure to low concentrations of silver ions, e.g. released by the AgNPs that end up in nature, could select for silver resistant bacteria, e.g. Randall et al. showed that E. coli can develop silver resistance in just six days when exposed to silver ions.26
An alternative method to physically remove bacteria from water, while keeping the filter pore size larger than bacteria, is to use positively charged filters that adsorb negatively charged bacteria onto the surfaces of the filters. This allows negatively charged particles much smaller than the filter pore size to be efficiently removed from water and this is an interesting approach for removing bacteria from water without adding any toxic chemicals or reducing the flow by reducing the pore size.27,28 Both Gram-positive and Gram-negative bacteria have a negative net surface charge on the cell envelope, due to peptidoglycans, liposaccharides and proteins in the cell wall, and this makes their removal non-selective and efficient for most types of bacteria.29–31
Surface grafting, by covalent linkage, is one of the more common ways to alter membrane surfaces.32 The surfaces of natural cellulose pulp fibres have a negative net charge but can be modified to obtain a positive surface charge.33 Recently, Peña-Gómez et al. have showed that it is possible to remove E. coli from water using amino-functionalized cellulose membranes, where a cationic polyamine has been covalently linked to paper filters using dimethyl sulfoxide (DMSO).34
Another way to change the surface charge of cellulose fibres, without using organic solvents, is to use polyelectrolyte adsorption. In this simple and environmentally sustainable process, cationic polyelectrolytes are physically adsorbed onto the fibre surface. To increase the surface coverage, the adsorption can be repeated in steps using alternating charged polyelectrolytes using the layer-by-layer (LbL) method.35–37 LbL modification amplifies the amount of cationic polymer adsorbed onto the substrate for each layer leading to a surface charge overcompensation.38–40 Cellulose fibres treated with multilayers of cationic polyvinyl amine (PVAm) and anionic polyacrylic acid (PAA) have previously been shown to adsorb more than 99.9% of E. coli in a fibre suspension without leaching any biocides.41–43 The effect was, however, notably reduced when turbid water samples were used, due to a decreased contact between the LbL-modified fibres and the bacteria, and a filtration approach is therefore desired.
Studies using LbL modification of filters for water purification application have so far been focused on nanofiltration either by creating thin film composites onto support membranes, thus reducing the pore size of the membranes, or creating antibacterial filters that release biocides. Liu et al. created antibacterial nanofiltration membranes using LbL with leaching AgNPs incorporated in the layer of the anionic polyelectrolyte to decrease biofouling of the membrane, and Imani et al. created antibacterial filter papers by alternating adsorption of cationic chitosan and AgNPs mixed in PAA.23,44 In this study, a previously used PVAm/PAA multilayer system is applied on two types of commercial cellulose filters to allow the trapping of bacteria through electrostatic interactions while keeping the large pores in the filters. Here, the LbL method has been utilized to create biobased filters for water purification without the addition of leaching biocides.
2. Materials and methods
2.1 Materials
Two different types of paper filters were selected for this study. A commercially available bleached cone coffee filter (FRIDA no. 4, Klimabolaget, Sweden), hereafter named filter A, with a thickness of 0.12 mm was selected to show the potential of the method to use readily available and affordable materials. Whatman qualitative filter paper (Grade 113, Sigma-Aldrich, Sweden) with high wet strength, a reported pore size of 30 μm and a thickness of 0.39 mm, hereafter named filter B, was selected as a well characterized filter with a known pore size.2.2 Chemicals
Cationic polyvinylamine Lupamin® 9095 (PVAm) with a molecular weight of 340 kDa supplied by BASF SE (Ludwigshafen, Germany) and anionic polyacrylic acid (PAA) with a reported molecular weight of 240 kDa obtained from Sigma-Aldrich (Stockholm, Sweden) were used for the LbL modifications.2.3 Methods
Escherichia coli (E. coli) ATCC 11775 (Sigma-Aldrich, Sweden), a biosafety level 2 microorganism, was prepared by cultivation overnight in an agitated nutrient broth medium (Scharlab, Barcelona, Spain) at 37 °C, before harvesting the bacteria through centrifugation, 5000g for 5 min, and re-suspension in ¼-strength Ringer's solution (Merck, Darmstadt, Germany). The harvested bacteria were washed once by a second centrifugation and re-suspension cycle.
The bacterial reduction efficiency of the filters was evaluated by filtering 10 mL of ¼-strength Ringer's solution with a bacterial concentration of 106 CFU mL−1 through the prepared filters. A sample of the filtrate, 1 mL, was spread and cultivated in duplicate on Petrifilm aerobic count plates (3M, Sollentuna, Sweden) to determine the number of viable bacteria remaining in the water after filtration, using the image analysis software ImageJ to count the CFU.45 All the reported results are averages of duplicate filtration processes. The water flow through the filters was caused by free flow filtration, due to gravity, or controlled with a programmable Aladdin syringe pump (World Precision Instruments, FL, USA), using autoclavable PharMed tubing with a diameter of 1.6 mm (Bio-Rad Laboratories, CA, USA) to connect a 10 mL syringe and the filter holder.
The bacteria-reducing performance when reusing the filters was evaluated by reusing a water filter, consisting of 20 sheets of the 3 L modified coffee filter, for five consecutive free flow filtration processes with a 106 CFU mL−1E. coli suspension. The filtrate was cultivated at 37 °C in duplicate on Petrifilm aerobic count plates to determine the number of viable bacteria remaining in the water.
Filters were prepared by assembling 15 sheets of filter A, either unmodified or 3 L modified, in syringe filter holders. The transformed fluorescent bacteria suspensions were prepared in ¼-strength Ringer's solution with a bacterial concentration of 106 CFU mL−1, and 10 mL of the bacterial suspension were filtered through the filters by free flow due to gravity. Fluorescence micrographs were taken on each layer in the stacked filter by carefully separating and transferring the sheets to microscopy slides without rinsing. The micrographs were captured using an LSM 510 Pascal inverted confocal laser scanning microscope (Zeiss, Oberkochen, Germany).
3. Results and discussion
Two types of paper filters, commercial coffee filter paper called filter A and Whatman filter paper called filter B, were modified with polyelectrolyte multilayers through LbL adsorption to obtain a positive net surface charge that can adsorb negatively charged bacteria. The bacterial removal efficiency of the modified filters was determined both under free flow filtration, due to gravity, and under controlled flow filtration using a syringe pump.3.1 Nitrogen content
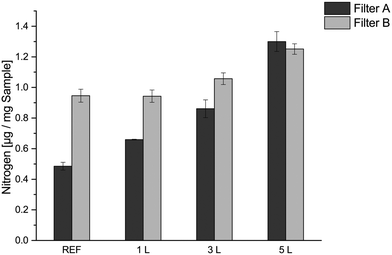
The nitrogen content analysis was performed to evaluate how much PVAm has been adsorbed onto the modified filter papers, as cationic PVAm is the component that provides the fibres with a positive surface charge. The nitrogen analysis showed that the nitrogen content in the filters increased with increasing number of PVAm layers and that the LbL modification alternating positively and negatively charged polyelectrolytes enhanced the amount of PVAm adsorbed onto the fibre surface (Fig. 1). A similar non-linear adsorption pattern for PVAm adsorption onto cellulose fibres has been reported by Illergård et al., where more PVAm were adsorbed for each added layer of PVAm.43 No significant difference in nitrogen content was seen between the unmodified filter B and the 1 L filter B, but the 1 L filter B later showed a good bacteria-reducing effect (Fig. 4). Filter B consisted of a wet strength agent containing nitrogen and a test was repeated with the washed unmodified reference with the same result.3.2 SEM
The structure of the filters before and after the 3 L LbL modification can be seen in Fig. 2. The cellulose fibres in filter A seem to be more tightly pressed than the fibres in filter B but no obvious difference in appearance can be observed between the unmodified and the 3 L LbL modified filters. Voids between the fibres can be seen in all four images. The voids between the fibres in filter A are in the size range of 50–90 μm while filter B shows larger voids between 50–150 μm, which should allow the bacteria that are approximately 1–2 μm in length to easily pass through the filters.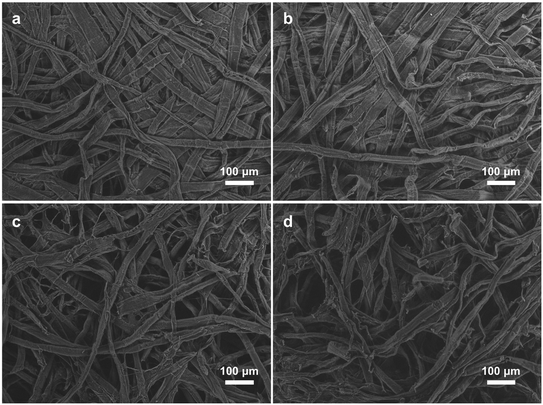 | ||
| Fig. 2 SEM images of the filters: a) unmodified filter A, b) 3 L LbL modified filter A, c) unmodified filter B and d) 3 L LbL modified filter B. | ||
3.3 Flow rate for free flow filtration
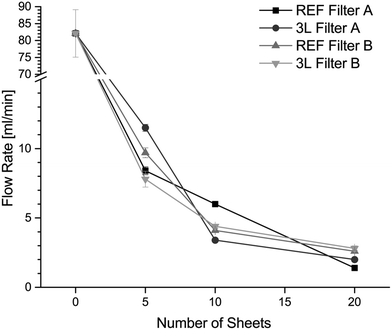
The flow rate test shows no obvious difference between the flow rate through the unmodified reference filter and that through the LbL modified paper filters. It can be clearly seen that the flow rate decreases with increasing number of sheets in the filter holder (Fig. 3). The filters with 20 paper sheets had a flow rate of approximately 2 mL min−1. There was no great difference in the flow rate between paper filters A and B, although the thicknesses of the filters were different. No obvious trend can be seen between the modified filters and the unmodified references. The multilayers of PVAm/PAA on the cellulose fibres in the filter should have no significant impact on the pore size in the filters as previous research studies have shown that bilayers of polyelectrolytes adsorbed by the LbL technique have a thickness in the nanometre range.35,393.4 Bacterial removal efficiency of filtration
The bacterial removal efficiency was evaluated by filtering 10 mL of 106 CFU mL−1E. coli suspension, with a flow rate of 1 mL min−1, through sheets of paper filters assembled in filter holders. A controlled water flow through the filters was achieved using a syringe pump. The high concentration of E. coli, compared to concentrations of faecal bacteria in real water samples that would be considered for drinking purposes, was used in these tests to show the potential of the LbL modified filters.The microbiological reduction requirement by the WHO on a highly protective household water treatment is the ability to remove ≥99.99% of E. coli from water, using an initial bacterial concentration of ≥103 CFU mL−1.47 A bacterial reduction of ≥99.0% is considered as the lowest limit for efficient bacterial removal, showing that the treatment method can significantly reduce bacteria. Additionally, the highly protective household water treatment method should also remove ≥99.999% of viruses and ≥99.99% of protozoa from water, which is not tested in this study.
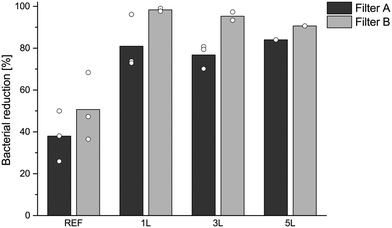
The bacterial removal efficiency of all three modifications was greater than that of the unmodified reference filters (Fig. 4). The reference sample with five sheets of unmodified filter A removed approximately 38% of the bacteria whereas the five sheets of filter A with the 1 L modification removed 81%, with the 3 L modification 77%, and with the 5 L modification 84% of the bacteria from water. The five sheets of unmodified filter B removed approximately 50% of the bacteria whereas the five sheets of 1 L modified filter B removed 98% and those of 3 L modified filter B removed 96% of the bacteria. The 5 L modified filter B removed 91%, which was less than those removed by the 1 L and 3 L modified filters, even though the 5 L modified filters contained more PVAm than the other modifications (Fig. 1). This is promising for a potential product, as fewer layers of polyelectrolytes simplify the modification process and reduce the amount of polymer needed and thus reduce the production costs. A previous study of the antibacterial effect of PVAm multilayers on different types of cellulose pulp fibres has shown that very small amounts of cationic PVAm are adsorbed on dissolving pulp, which has a low total fibre charge, but the modified dissolving pulp still showed a good bacteria-reducing effect.48 The bacteria-reducing effect is however not only dependent on the amount of adsorbed PVAm, as some of the cationic charges are consumed when building multilayers and the amount of cationic charges available for contact with bacteria could be crucial for the antibacterial effect.40 The filtration tests were thus focused on the 1 L and 3 L LbL modifications, as they had the best removal efficiency taking into consideration the amount of polymer and the number of process steps needed for the fibre modification.
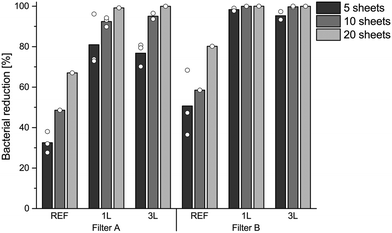
The next filtration test evaluated the dependence of the removal efficiency on the number of paper sheets used for the filtration and it was found that the bacterial reduction can be greatly improve by optimizing the design of the filter. The bacterial reduction efficiency of the LbL modified filters increases with increasing the number of sheets used (Fig. 5). The bacterial removal was greater than 99.9%, corresponding to a 3 log10 reduction, when the bacterial solution was filtered through 20 sheets of 3 L LbL modified filter A (coffee filter) or filter B (Whatman filter), as well as 10 sheets of 1 L modified filter B. It is thus possible to improve the bacteria-reducing effect by increasing the number of sheets in the filter. The difference between filter A and filter B was most noticeable when using 5 and 10 sheets of filter paper, probably in part because of the difference in the thickness of the filters. The reduction values of 99.9% are higher than the WHO's lowest limits for pathogen bacterial removal using household water treatment methods, ≥99.0%, and a 10 times greater removal efficiency is required to reach the standards for very high pathogen removal.47
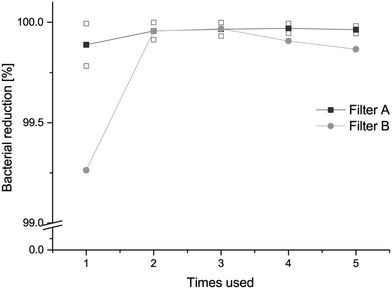
The dependence of the bacteria-reducing performance of the filter on the number of times the filter was used was evaluated by reusing the filters for five consecutive free flow filtration processes. The bacterial suspensions were filtered through 20 sheets of 3 L modified filter A or filter B. The bacterial reduction capacity was greater than 99.9% for the 2nd to the 5th filtration (Fig. 6). The first filtration showed a slightly smaller reduction of bacteria for both filter A and filter B than the later subsequent four filtration processes. This may be due to the dry state of the filters for the first filtration. The polyelectrolytes need to be in a wet environment to be fully charged and the pores of the filter probably shrink slightly when the fibre absorbs water into the cell wall and the fibre thickness increases.49 The bacterial removal effect of contact-active materials is known to be dependent on the applied bacterial load and the bacterial adsorption is limited to the available charges on the modified fibres.27 It is nevertheless evident that it is possible to reuse the filter several times with the same bacterial-removal effect.
3.5 Filtration of natural water samples
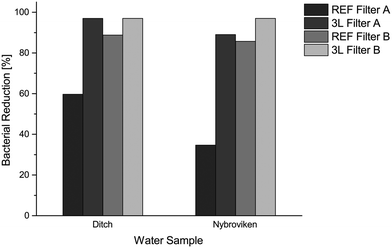
The filtration test with real water samples showed that the polyelectrolyte modified filters are efficient in removing complex mixtures of microorganisms from natural water samples. The ditch water contained approximately twice as much bacteria, detectable by cultivation, as the Nybroviken water sample. The Nybroviken water contained approximately 1 × 103 CFU mL−1 and the ditch water 2.5 × 103 CFU mL−1, which were approximately 103 times less bacteria than that used in the tests with pure E. coli suspensions. Microorganisms from environmental samples are however difficult to cultivate in the laboratory; it has been shown that only a fraction of the bacteria present in samples from nature can be cultivated in a laboratory environment.50,51 It should be noted that the bacteria from the natural water samples were cultivated at 37 °C, in consistency with the other experiments, which may not be an ideal temperature for all species in the samples.The unmodified filters had a moderate bacteria-reducing effect, as some bacteria were retained in the filter and particles in the water hosting bacteria were removed through size exclusion, but the 3 L LbL modified filters showed a much greater bacteria-reducing effect than the unmodified reference filters. Cultivations with colonies less than 30 CFU are generally considered to be too few to count (TFTC), which gives a maximum detectable reduction of 97% for the natural water samples.52 The 20 sheets of 3 L filter A removed over 97% of the bacteria from the ditch water and 89% from the Nybroviken water, while 10 sheets of 3 L filter B removed over 97% from both the ditch water and the Nybroviken water samples (Fig. 7). The filtration mode enables removal of particles that can host bacteria and interfere with the bacterial adsorption in the natural water samples and the filtration test shows an improvement in both time consumption and decontamination efficiency compared to previous studies using LbL-modified pulp fibres freely dispersed in natural water samples.53
3.6 Fluorescence microscopy images
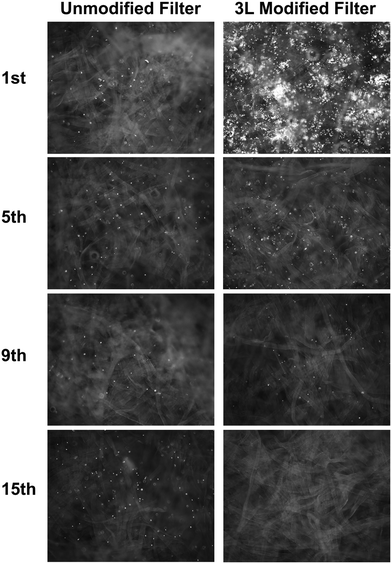
The bacterial retention in the water filters was studied by filtering a suspension of fluorescent E. coli through 15 sheets of unmodified and 3 L modified filter A. The micrographs of the sheets after filtration, captured by fluorescence microscopy, show the cellulose fibres, which are slightly auto-fluorescent, as well as the fluorescent E. coli.The micrographs of the unmodified paper filter show no difference between the sheets in the stack of filter paper (Fig. 8). The bacteria seemed to be evenly distributed throughout the unmodified filter as white dots, which was expected since the filtration test showed no great reduction of bacteria by the unmodified filter A. The bacteria seen on the unmodified sheets seemed to be free in suspension and were seen floating around the fibres, while the bacteria on the 3 L modified filters seemed to be fixed onto the fibres. The bacteria on the first sheet of filter A were unevenly adsorbed onto the cellulose fibres. This type of bacterial adsorption in islands has been previously seen with the same modification on films made from CNFs; Henschen et al. showed a higher concentration of adsorbed bacteria in the ridges on a structured CNF film modified with LbL and it was suggested to be due to the difference in the structure and roughness between the ridges and the flat parts of the CNF film.54 The fluorescence micrographs show that most bacteria were adsorbed in the first sheet of the stacked filter when 3 L modification was applied to the cellulose filters. Fewer bacteria were seen further down in the modified filter stack. A couple of bacteria can be seen on the 9th sheet but the bottom 15th sheet shows no fluorescent bacteria. This supports the findings that more sheets in the filter improve the bacteria-reducing effect and that it should be possible to optimize the filtration to improve the bacterial reduction even further.
4. Conclusion
This study has investigated the bacterial removal efficiency of cellulose-based paper filters modified by LbL adsorption of PVAm and PAA, which could be an affordable and environmentally friendly alternative for disposable POU water purification to remove bacteria from water e.g. in emergency situations to prevent diseases. The antibacterial effect of the modified filters is based on a contact-active approach to physically adsorb and remove bacteria from water through electrostatic interactions without leaching biocides into the water or noticeably reducing the pore size of the filter. A great advantage of contact-active bacterial removal compared to POU filters using biocides such as AgNPs or chlorine is that the decontamination process is complete as soon as the water has penetrated the filters. No extra time for bacteria inactivation is needed, which is the case when using biocides that need more or less time to inactivate the bacteria.The use of LbL modified paper filters were shown to be an efficient and fast way to remove bacteria from contaminated waters, both when it comes to high concentrations of E. coli and the complex microbial mixtures in real water samples. Three types of polyelectrolyte modifications have been tested: 1 L, 3 L and 5 L, and it is shown that 1 L and 3 L performed as good as or better than the 5 L modification, containing the highest amount of PVAm. It is possible to remove over 97% of the cultivatable bacteria in real water samples from nature when using 3 L modified paper filters in filtration mode, and 20 sheets of 3 L modified paper filter A removed over 99.9% of the E. coli from water, while 20 sheets of filter B could remove over 99.9% of the bacteria using both the 1 L and 3 L modifications. The filters can be reused several times and it is shown that most of the bacteria adsorb onto the cellulose fibres in the first few sheets in the 3 L modified water filter stack, while they pass through the unmodified filters without any major reduction.
The LbL modification used to create bacteria adsorbing surfaces is an interesting and promising technique that could potentially be used to make bio-based filters for water purification in the future. Further research and improvements of the filters and the filtration design are needed to reach a high pathogen removal classification, according to the WHO's recommended standards for household water treatment.47
Abbreviations
| AgNPs | Silver nanoparticles |
| CFU | Colony forming units |
| CuNPs | Copper nanoparticles |
| LbL | Layer-by-layer |
| PAA | Polyacrylic acid |
| POU | Point-of-use |
| PVAm | Polyvinylamine |
| SEM | Scanning electron microscope |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the ÅForsk Foundation [grant no. 17-391] and the Royal Swedish Academy of Agriculture and Forestry [grant no. GFS2017-0107]. The authors thank the foundations for supporting the research and BASF for supplying the PVAm used in this study.References
- J. Holmgren, L. Bourgeois, N. Carlin, J. Clements, B. Gustafsson, A. Lundgren, E. Nygren, J. Tobias, R. Walker and A. M. Svennerholm, Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant, Vaccine, 2013, 31, 2457–2464 CrossRef CAS PubMed.
- WHO and UNICEF, Progress on sanitation and drinking water–2015 update and MDG assessment, World Health Organization, 2015 Search PubMed.
- Z. A. Bhutta and J. K. Das, Global Burden of Childhood Diarrhea and Pneumonia: What Can and Should Be Done?, Pediatrics, 2013, 131, 634–636 CrossRef PubMed.
- T. A. Dankovich and D. G. Gray, Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment, Environ. Sci. Technol., 2011, 45, 1992–1998 CrossRef CAS PubMed.
- M. D. Sobsey, C. E. Stauber, L. M. Casanova, J. M. Brown and M. A. Elliott, Point of Use household drinking water filtration: A practical, effective solution for providing sustained access to safe drinking water in the developing world, Environ. Sci. Technol., 2008, 42, 4261–4267 CrossRef CAS PubMed.
- S. Doocy and G. Burnham, Point-of-use water treatment and diarrhoea reduction in the emergency context: an effectiveness trial in Liberia, Trop. Med. Int. Health, 2006, 11, 1542–1552 CrossRef PubMed.
- K. Bethke, S. Palantöken, V. Andrei, M. Roß, V. S. Raghuwanshi, F. Kettemann, K. Greis, T. T. Ingber, J. B. Stückrath and S. Valiyaveettil, Functionalized Cellulose for Water Purification, Antimicrobial Applications, and Sensors, Adv. Funct. Mater., 2018, 28, 1800409 CrossRef.
- H. K. Lonsdale, The growth of membrane technology, J. Membr. Sci., 1982, 10, 81–181 CrossRef CAS.
- K. J. Howe and M. M. Clark, Fouling of microfiltration and ultrafiltration membranes by natural waters, Environ. Sci. Technol., 2002, 36, 3571–3576 CrossRef CAS PubMed.
- B. P. Tripathi, N. C. Dubey and M. Stamm, Functional polyelectrolyte multilayer membranes for water purification applications, J. Hazard. Mater., 2013, 252–253, 401–412 CrossRef CAS PubMed.
- A. W. Carpenter, C.-F. O. de Lannoy and M. R. Wiesner, Cellulose nanomaterials in water treatment technologies, Environ. Sci. Technol., 2015, 49, 5277–5287 CrossRef CAS PubMed.
- H. Voisin, L. Bergström, P. Liu and A. P. Mathew, Nanocellulose-based materials for water purification, Nanomaterials, 2017, 7, 57 CrossRef PubMed.
- H. Ma, C. Burger, B. S. Hsiao and B. Chu, Ultra-fine cellulose nanofibers: new nano-scale materials for water purification, J. Mater. Chem., 2011, 21, 7507–7510 RSC.
- A. Mautner, K.-Y. Lee, T. Tammelin, A. P. Mathew, A. J. Nedoma, K. Li and A. Bismarck, Cellulose nanopapers as tight aqueous ultra-filtration membranes, React. Funct. Polym., 2015, 86, 209–214 CrossRef CAS.
- A. W. Zularisam, A. F. Ismail and R. Salim, Behaviours of natural organic matter in membrane filtration for surface water treatment — a review, Desalination, 2006, 194, 211–231 CrossRef CAS.
- W. L. Chou, D. G. Yu and M. C. Yang, The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment, Polym. Adv. Technol., 2005, 16, 600–607 CrossRef CAS.
- S. F. Thomas, P. Rooks, F. Rudin, S. Atkinson, P. Goddard, R. Bransgrove, P. T. Mason and M. J. Allen, The bactericidal effect of dendritic copper microparticles, contained in an alginate matrix, on Escherichia coli, PLoS One, 2014, 9, e96225 CrossRef PubMed.
- T. A. Dankovich, J. S. Levine, N. Potgieter, R. Dillingham and J. A. Smith, Inactivation of bacteria from contaminated streams in Limpopo, South Africa by silver-or copper-nanoparticle paper filters, Environ. Sci.: Water Res. Technol., 2016, 2, 85–96 RSC.
- T. A. Dankovich and J. A. Smith, Incorporation of copper nanoparticles into paper for point-of-use water purification, Water Res., 2014, 63, 245–251 CrossRef CAS PubMed.
- K. Gopal, S. S. Tripathy, J. L. Bersillon and S. P. Dubey, Chlorination byproducts, their toxicodynamics and removal from drinking water, J. Hazard. Mater., 2007, 140, 1–6 CrossRef CAS PubMed.
- B. Ehdaie, C. Krause and J. A. Smith, Porous ceramic tablet embedded with silver nanopatches for low-cost point-of-use water purification, Environ. Sci. Technol., 2014, 48, 13901–13908 CrossRef CAS PubMed.
- K. Mijnendonckx, N. Leys, J. Mahillon, S. Silver and R. Van Houdt, Antimicrobial silver: uses, toxicity and potential for resistance, BioMetals, 2013, 26, 609–621 CrossRef CAS PubMed.
- R. Imani, M. Talaiepour, J. Dutta, M. R. Ghobadinezhad, A. H. Hemmasi and M. M. Nazhad, Production of antibacterial filter paper from wood cellulose, BioResources, 2011, 6, 891–900 Search PubMed.
- K. Levy, L. Anderson, K. A. Robb, W. Cevallos, G. Trueba and J. N. S. Eisenberg, Household effectiveness vs. laboratory efficacy of point-of-use chlorination, Water Res., 2014, 54, 69–77 CrossRef CAS PubMed.
- R. J. Griffitt, R. Weil, K. A. Hyndman, N. D. Denslow, K. Powers, D. Taylor and D. S. Barber, Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio), Environ. Sci. Technol., 2007, 41, 8178–8186 CrossRef CAS PubMed.
- C. P. Randall, A. Gupta, N. Jackson, D. Busse and A. J. O'neill, Silver resistance in Gram-negative bacteria: a dissection of endogenous and exogenous mechanisms, J. Antimicrob. Chemother., 2015, 70, 1037–1046 CAS.
- W. Ho and K. Sirkar, Membrane handbook, Springer Science & Business Media, 2012 Search PubMed.
- K.-M. Yao, M. T. Habibian and C. R. O'Melia, Water and waste water filtration: concepts and applications, Environ. Sci. Technol., 1971, 5, 1105–1112 CrossRef CAS.
- J. A. Lichter, K. J. Van Vliet and M. F. Rubner, Design of antibacterial surfaces and interfaces: polyelectrolyte multilayers as a multifunctional platform, Macromolecules, 2009, 42, 8573–8586 CrossRef CAS.
- Y. Hong and D. G. Brown, Electrostatic behavior of the charge-regulated bacterial cell surface, Langmuir, 2008, 24, 5003–5009 CrossRef CAS PubMed.
- A. van der Wal, W. Norde, A. J. B. Zehnder and J. Lyklema, Determination of the total charge in the cell walls of Gram-positive bacteria, Colloids Surf., B, 1997, 9, 81–100 CrossRef.
- J. Saqib and I. H. Aljundi, Membrane fouling and modification using surface treatment and layer-by-layer assembly of polyelectrolytes: state-of-the-art review, J. Water Process Eng., 2016, 11, 68–87 CrossRef.
- T. Lindström and C. Söremark, Adsorption of cationic polyacrylamides on cellulose, J. Colloid Interface Sci., 1976, 55, 305–312 CrossRef.
- N. Peña-Gómez, M. Ruiz-Rico, I. Fernández-Segovia and J. M. Barat, Development of amino-functionalized membranes for removal of microorganism, Innovative Food Sci. Emerging Technol., 2018, 48, 75–82 CrossRef.
- G. Decher, J. D. Hong and J. Schmitt, Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces, Thin Solid Films, 1992, 210–211, 831–835 CrossRef CAS.
- L. Wågberg, S. Forsberg, A. Johansson and P. Juntti, Engineering of fibre surface properties by application of the polyelectrolyte multilayer concept. Part I: Modification of paper strength, J. Pulp Pap. Sci., 2002, 28, 222–228 Search PubMed.
- M. Eriksson, S. M. Notley and L. Wågberg, The influence on paper strength properties when building multilayers of weak polyelectrolytes onto wood fibres, J. Colloid Interface Sci., 2005, 292, 38–45 CrossRef CAS PubMed.
- C. Picart, J. Mutterer, L. Richert, Y. Luo, G. Prestwich, P. Schaaf, J.-C. Voegel and P. Lavalle, Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12531–12535 CrossRef CAS PubMed.
- G. Decher, Fuzzy nanoassemblies: toward layered polymeric multicomposites, Science, 1997, 277, 1232–1237 CrossRef CAS.
- J. B. Schlenoff and S. T. Dubas, Mechanism of polyelectrolyte multilayer growth: charge overcompensation and distribution, Macromolecules, 2001, 34, 592–598 CrossRef CAS.
- J. Illergård, L. Wågberg and M. Ek, Contact-active antibacterial multilayers on fibres: a step towards understanding the antibacterial mechanism by increasing the fibre charge, Cellulose, 2015, 22, 2023–2034 CrossRef.
- J. Illergård, L. Wågberg and M. Ek, Bacterial-growth inhibiting properties of multilayers formed with modified polyvinylamine, Colloids Surf., B, 2011, 88, 115–120 CrossRef PubMed.
- J. Illergård, U. Römling, L. Wågberg and M. Ek, Biointeractive antibacterial fibres using polyelectrolyte multilayer modification, Cellulose, 2012, 19, 1731–1741 CrossRef.
- X. Liu, S. Qi, Y. Li, L. Yang, B. Cao and C. Y. Tang, Synthesis and characterization of novel antibacterial silver nanocomposite nanofiltration and forward osmosis membranes based on layer-by-layer assembly, Water Res., 2013, 47, 3081–3092 CrossRef CAS PubMed.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
- M. Mandel and A. Higa, Calcium-dependent bacteriophage DNA infection, J. Mol. Biol., 1970, 53, 159–162 CrossRef CAS PubMed.
- WHO, Results of round I of the WHO international scheme to evaluate household water treatment technologies, 2016 Search PubMed.
- J. Illergård, U. Römling, L. Wågberg and M. Ek, Tailoring the effect of antibacterial polyelectrolyte multilayers by choice of cellulosic fiber substrate, Holzforschung, 2013, 67, 573–578 Search PubMed.
- S. Fält, L. Wågberg and E.-L. Vesterlind, Swelling of model films of cellulose having different charge densities and comparison to the swelling behavior of corresponding fibers, Langmuir, 2003, 19, 7895–7903 CrossRef.
- R. I. Amann, W. Ludwig and K.-H. Schleifer, Phylogenetic identification and in situ detection of individual microbial cells without cultivation, Microbiol. Rev., 1995, 59, 143–169 CAS.
- T. Kaeberlein, K. Lewis and S. S. Epstein, Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment, Science, 2002, 296, 1127–1129 CrossRef CAS PubMed.
- G. O'Toole, Classic spotlight: plate count you can count on, J. Bacteriol., 2016, 198, 3127 CrossRef PubMed.
- A. Ottenhall, J. Illergård and M. Ek, Water purification using functionalized cellulosic fibers with non-leaching bacteria adsorbing properties, Environ. Sci. Technol., 2017, 51, 7616–7623 CrossRef CAS PubMed.
- J. Henschen, P. A. Larsson, J. Illergård, M. Ek and L. Wågberg, Bacterial adhesion to polyvinylamine-modified nanocellulose films, Colloids Surf., B, 2017, 151, 224–231 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |