The fate of dichloroacetonitrile in UV/Cl2 and UV/H2O2 processes: implications on potable water reuse†
Abstract
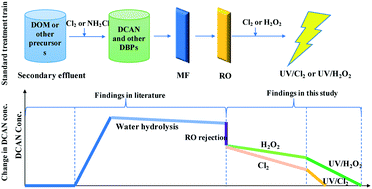
This study investigated the fate of DCAN in UV/Cl2 and UV/H2O2 processes under the conditions relevant to potable water reuse (e.g., two pH values and three oxidant dosages). At pH 6 and an oxidant dosage of 500 μM, the degradation of DCAN in the UV/Cl2 process was attributed to UV photolysis (4.5%), HO−-assisted hydrolysis (10.5%), nucleophilic attack by ClO− (32.2%), and oxidation by radicals (i.e., HO˙ and Cl˙) (52.8%), while that in the UV/H2O2 process was mainly attributed to HO2− (32%) and HO˙ (48%). In both processes, the DCAN degradation rates were higher with increasing solution pH from 5 to 6, because the increased HO−-assisted hydrolysis and nucleophilic attack of DCAN surpassed the decreased radical oxidation of DCAN. The DCAN degradation was enhanced with increasing chlorine or H2O2 dosage from 100 to 1000 μM, mainly due to the increased contribution from the nucleophilic attack. DCAN was mostly transformed into dichloroacetic acid (DCAA) through different pathways in the two processes. At the same pH and oxidant dosage conditions, the degradation rates of DCAN in the UV/Cl2 process were higher than those in the UV/H2O2 process, due to the higher nucleophilic attack rates and radical oxidation rates in the former process. The cost of degrading 90% of DCAN using the UV/Cl2 process is about 1/8 of that using the UV/H2O2 process, making the UV/Cl2 process a more cost-effective UV-AOP in DCAN abatement for potable water reuse.
- This article is part of the themed collections: Best Papers 2018 – Environmental Science: Water Research & Technology and Ultraviolet-based Advanced Oxidation Processes (UV AOPs)


 Please wait while we load your content...
Please wait while we load your content...