Cyto- and geno-toxicity of 1,4-dioxane and its transformation products during ultraviolet-driven advanced oxidation processes†
Abstract
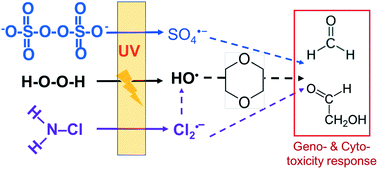
Ultraviolet-driven advanced oxidation processes (UV/AOPs) are integral steps in water reuse treatment trains. The toxicity of trace organic transformation products during a UV/AOP is critical to its implementation. This study examined the cyto- and geno-toxicity of transformation products of 1,4-dioxane (1,4-D), a trace organic contaminant commonly found in secondary wastewater, in extracts using the CellSensor p53RE-bla HCt-116 cell assay, following UV photolysis at 254 nm with three oxidants, hydrogen peroxide (H2O2), persulfate (S2O82−) and monochloramine (NH2Cl). 1,4-D was transformed into six major oxidation by-products, including ethylene glycol diformate, formaldehyde, glycolaldehyde, glycolic acid, formic acid, and methoxyacetic acid. Formaldehyde and glycolaldehyde were the most geno- and cyto-toxic, while 1,4-D had weak genotoxicity and no cytotoxicity. The order for cytotoxicity on the basis of EC50 values is as follows: glycolaldehyde > formaldehyde > formic acid > glycolic acid > 1,4-D > ethylene glycol diformate ≈ methoxyacetic acid, with glycolaldehyde and formaldehyde showing high genotoxicity. With the three UV/AOPs, genotoxicity expressed as mitomycin equivalency quotient (MEQ) increased significantly by 10 to 100 fold with a UV dosage of 720 mJ cm−2, mainly due to the formation of glycolaldehyde. UV/S2O82− reduced the MEQ with an increased UV dosage of 1440 mJ cm−2, due to the transformation of toxic aldehydes to less toxic organic acids. In contrast, UV/H2O2 increased the MEQ with UV dosage, resulting from the accumulation of aldehyde products. UV/NH2Cl showed the lowest MEQ due to its slow removal of 1,4-D. This study suggests that oxidants and UV dosage can affect the toxicological responses of treatments for recycled water.
- This article is part of the themed collections: Best Papers 2018 – Environmental Science: Water Research & Technology, Best Papers from 2018 in the Environmental Science Family of Journals and Ultraviolet-based Advanced Oxidation Processes (UV AOPs)


 Please wait while we load your content...
Please wait while we load your content...