Facet-dependent contaminant removal properties of hematite nanocrystals and their environmental implications
Abstract
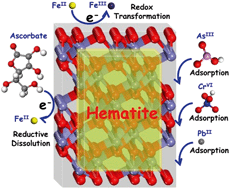
Hematite nanocrystals are ubiquitous in natural environments, and because of their strong sorption properties these particles can influence the mobility and fate of toxic elements such as hexavalent chromium and arsenic. It is now well established that sorption onto hematite is morphology dependent, because its hexagonal structure imparts distinct interfacial chemistry to individual facets. It is also well known that interaction of these facets with ferrous iron can impart powerful catalytic reduction capacity at these interfaces. However, the underlying mechanisms for facet-specific sorption and reductive transformation of specific metals remains poorly understood at the molecular scale. In this article, we first briefly review the synthesis of size and morphologically well-defined hematite nanocrystals and their corresponding growth mechanisms. We then summarize recent advances in understanding interactions between hematite facets and ferrous iron, as well as their impact on the geochemical cycles of some elements and contaminants. We emphasize the reductive dissolution of hematite facets and elucidate corresponding environmental implications. Furthermore, we focus on facet-dependent adsorption of model environmental contaminants. Finally, we highlight the importance of hematite nanocrystals for remediation and provide suggestions for improving understanding of their roles in the environment.


 Please wait while we load your content...
Please wait while we load your content...