 Open Access Article
Open Access ArticleToward computational and experimental characterisation for risk assessment of metal oxide nanoparticles
Laura
Escorihuela
 a,
Benjamí
Martorell
a,
Benjamí
Martorell
 *a,
Robert
Rallo
*a,
Robert
Rallo
 b and
Alberto
Fernández
b and
Alberto
Fernández
 a
a
aDepartament d'Enginyeria Química, Universitat Rovira i Virgili, Avinguda dels Països catalans, 26, 43007 Tarragona, Spain. E-mail: benjami.martorell@urv.cat
bAdvanced Computing, Mathematics, and Data Division, Pacific Northwest National Laboratory, Richland, WA 99352, USA
First published on 7th September 2018
Abstract
Metal oxide (MeO) nanoparticles (NPs) have become common in our everyday life over the past years. However, there is still an important knowledge gap regarding their toxicological effect and, in particular, how the different physical and chemical properties of MeO NPs influence their cytotoxicity and the subsequent implications for risk assessment. This work analyses the physicochemical properties of MeO NPs that have been reported as relevant for risk assessment and the experimental and theoretical methods used to obtain them. The surface, physical and chemical properties of NPs have been critically revisited to shed light on the features that can cause toxicity. Due to the large number of existing MeO NPs, in silico studies are necessary to get a good understanding of the NPs' physicochemical properties; therefore this review focuses on the state of the art computational methods used to model MeO NP toxicity: QSAR and QSTR models and their alternative approaches provide a better understanding of MeO NP biological toxicity in organisms.
Environmental significanceThe use of nanoparticulate materials has exponentially grown during this decade due to their extraordinary properties, covering a wide range of products in the opto-electronics, pharmaceutical, medical, cosmetic and textile industries. However, the risk to human health (cytotoxic, mutagenic or carcinogenic effects) for most of them is still not well established. Alternative routes for risk assessment based on in silico methods avoid the highly expensive and time-consuming toxic evaluation of nanoparticles in the laboratory. Cheminformatic tools relate physicochemical properties with the cytotoxic effects, but standardised methods are missing. Therefore, the establishment of standard properties and mathematical models to predict toxicity is necessary for a more efficient assessment of nanoparticles. |
1. Introduction
The Nobel prize awardee Richard Phillips Feynman, with his famous sentence in 1959 stating that “there's plenty of room at the bottom”,1 was the first to envisage the potential of nanoscale materials for the global progress of industrial society and technology. In Europe, nanotechnology is considered as a key enabling technology (KET) that provides the basis for new advances and innovations in many fields of science and technology. In terms of economic impact, the global market of nano-enabled products was valued at $26 billion in 2014 and is expected to reach about $64.2 billion by 2019.2Particles with one or more of their dimensions in the range of a few nm up to tenths of μm have different properties, effects and behaviour relative to their microscale counterparts.3 Recent studies provided more insight on the size dependence of nanoparticle4 properties and reactivity, revealing that small sized nanoparticles (NPs) have a more variable behaviour in terms of their properties than larger size NPs, which have a more constant behaviour. For example, size dependence changes in NPs below 5 nm have more influence than changes in NPs in the range of 15 to 90 nm due to the quantum size and macro-quantum tunnelling effects.5 Another relevant effect of the smaller NPs is the direct exposure in an organism via the mechanism of entering directly inside the body and dissolving and delivering the toxic metal, described as the Trojan effect. This effect is specific for nanoscale particles given the inadvertent recognition by cell receptors.6
Due to their intrinsic properties, nanomaterials (NMs) are the cornerstone of a wide range of technologically advanced applications, with metal oxide (MeO) NPs being the most used in areas such as electronics, optics, opto-electronics, pharmacy, medicine, cosmetics and textiles.1,5 NPs are becoming more and more common in many consumer products; however there is still an important knowledge gap regarding how size influences their physicochemical properties and, in turn, their toxicity.7,8 For instance, at the nanoscale, several metal oxides are toxic, whereas they do not show any significant toxicity at the microscale.9 In 2006, Nel et al. described the mechanisms used by NMs to interact with biological systems and their toxicological effects; since then, their total comprehension has not been achieved yet.6
Therefore, NMs need a specific regulation to assess their toxicity. In the EU, the REACH10 (Registration, Evaluation, Authorisation and Restriction of Chemicals) agency directive is the current regulatory framework for chemical risk assessment and management. NMs are considered as independent “chemical substances” and therefore their registration and labelling are also regulated. The EU acknowledges that the application of REACH may cause administrative burden, affect time to market and increase marginal costs of nano-enabled products and technologies. In the United States, the Environmental Protection Agency (EPA) has a special regulation for NMs, the Toxic Substances Control Act (TSCA). NMs are referred to in TSCA as chemicals at the nanoscale. Due to their increased use in a huge range of products, in 2015 the TSCA regulation was extended to include chemical substances manufactured or processed as nanoscale materials (https://www.epa.gov/reviewing-new-chemicals-under-toxic-substances-control-act-tsca/control-nanoscale-materials-under).
Nonetheless, toxicity assessment of NMs is a daunting task that involves multiple testing conditions and endpoints, and testing of different NP configurations (i.e., different combinations of core, shell and functionalization layers, etc.). In silico testing, specifically the establishment of quantitative (nano)structure–activity relationships (QNARs), nano-quantitative structure–property relationships (nano-QSPRs) or quantitative structure–toxicity relationships (QSTRs), constitutes a cost-effective approach to fill the existing gaps in nanosafety data. The establishment of nano-QSPRs and QNARs requires (i) a detailed physicochemical and biological characterization of NMs and (ii) the development of computational nano-descriptors suitable to represent the electronic, atomic and molecular structures of NMs.
The development and validation of standard protocols for the experimental and theoretical characterization of NPs is fundamental to the generation of the high-quality data required to develop reliable nano-QSPRs and QNARs. Several reference descriptions of experimental and theoretical research protocols have been published by the Organisation for the Economic Cooperation and Development (OECD).11 In addition, the Nanosafety Cluster,12 promoted by the EU commission, helps to monitor and harmonize the European activities related to the risk assessment of NMs.
Recent results have described mathematical models linking NM structure descriptors with toxicity effects. These descriptors include physical and chemical properties such as electronic band gap, or surface properties such as surface formation energy or reactive sites.8,13,14 Regarding this relationship, for example, a band gap descriptor can be used to estimate the oxidative stress of MeO NPs.15 Recent studies separate the surface modifiers of NPs from the core of a MeO for predicting cellular uptake.16 In particular, QSAR models show that diverse combinations of NP properties can be used to classify different levels of biological response for ZnO and TiO2 NPs.17
Regarding the use of theoretical testing methods, REACH promotes the use of computational methods to implement 3R (replacement, reduction and refinement) approaches aimed at reducing and ultimately avoiding animal testing. Furthermore, REACH considers a “chemical element obtained by any manufacturing process, including any impurity deriving from the process used”. Any chemical element can be classified within different levels of impurities if it provides hazardous properties. Therefore, REACH forces NP producers and importers to provide toxicological data and environmental impact assessments (e.g., environmental exposure) when the NP concentrations are lower than 0.1% in weight. The effective implementation of REACH regulations requires the development of alternative non-testing methods (e.g., QSAR) to evaluate the toxicity of nanoparticles.
This review discusses relevant physicochemical properties of metal oxide nanoparticles and their implications for in silico nanotoxicity assessment of MeO NPs based on OECD recommendations. It is focused on the description of special properties of MeO NPs, their implications for risk assessment and their use in nano-QSAR and nano-QSPR methods for toxicity prediction. Finally, potential future research directions and challenges are discussed.
2. Metal oxide nanoparticles
MeO NPs are widely used in many technological applications such as semiconductors, capacitors, coatings, solar cells, etc. because of their highly efficient properties due to their limited size and high density of corner or edge surfaces, that result in unique optical, chemical sensing, and semiconducting properties. However, the high diversity of MeO and sizes of NPs and the lack of standardized measurement protocols hinders the use of physicochemical parameters for risk assessment.Bulk oxides are stable in a well-defined solid crystallographic structure (or a few structures, in some cases) under standard conditions. However, for smaller sizes (e.g. microscopic scale), the lattice stress must be taken into account because it can affect the structural properties up to the total disappearance of the crystallographic structure at the NP limit. Accordingly, phases with low stability in bulk form can be found at the nanoscale. This structural phenomenon has been reported for TiO2, VOx, Al2O3 and MoOx oxides.6
Nanoparticle size also influences other important features of electronic and physicochemical properties such as electrical conductivity and colour. At the nanoscale, semiconducting materials become metallic and non-magnetic particles become magnetic due to quantum-size and macro-quantum tunnelling effects. From the point of view of solid-state physics, both the superposition of bulk states and the increase in the material strength may affect electronic properties such as the band gap.
This section discusses and analyses the physicochemical properties of NPs, identified by the OECD18 as relevant for risk assessment, that are affected by the size or by the special reactivity of MeO NPs.
2.1. Surface properties
Surface properties play an important role in many aspects of material applications. Top layers of atoms in surfaces come into direct contact with their surrounding environment. Based on their applicability to risk assessment, surface properties and reactivity are considered as key nanoparticle features in the OECD test guidelines.11 Surface properties are influenced not only by the external layer of atoms but also by some internal layers.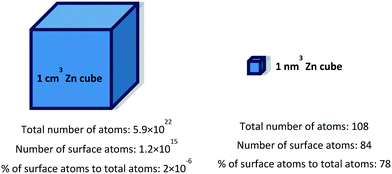 | ||
| Fig. 1 Comparison of the percentage of atoms exposed on the surface at macro and nano scales. Adapted from the book Science at the Nanoscale.19 | ||
Surface atoms are not completely coordinated with respect to the interior atoms, and therefore they show a higher energy and reactivity than the fully coordinated ones. The extra energy at the surface, γ, is defined as the free energy necessary to create a new unit of area:19
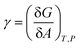 | (1) |
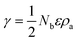 | (2) |
From the perspective of nanoparticle characterisation, surface charge is usually reported as the zeta potential, which includes the electric potential in the interfacial double layer and the pKa of the particle.20 As a consequence, this property is a good toxicity predictor for low-solubility NPs, and it is considered by the OECD as a relevant parameter for fate and exposure evaluation.22 Results have confirmed that the zeta potential is affected not only by suspension conditions such as pH, temperature, ionic strength and the types of ions in suspension23 but also by intrinsic particle properties such as size and concentration. Nevertheless, these data are not always well reported in the bibliography, given that sometimes the variability of the experimental process is not described in detail.24
The zeta potential is usually measured using particle size analyzers based on laser Doppler electrophoresis. The zeta potential, ξ, is calculated from the measured electrophoretic mobility, μ, through Henry's approximation:23
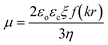 | (3) |
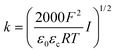 | (4) |
The influence of pH on the zeta potential is explained by the protonation/deprotonation of oxides and metal centres on the surface groups of the MeO NPs. MeO NPs have a positive zeta potential in acidic environments, whereas in basic environments the property has negative values.22 For example, in the case of bare TiO2 at pH 6.0, ξ increases from 6.7 to 8.2 mV as the particle concentration varies from 0.5 to 5.0 mg L−1.
Several studies also revealed that the zeta potential could depend on particle concentration.23,25 For example, as the particle concentration increases from 1.0 to 10.0 mg L−1, the zeta potential of naked TiO2 at pH 6.0 varies from 6.7 to 11.7 mV, and from 4.7 to 10.3 mV for Fe(OH)3 NPs at pH 7.5. This effect, however, could be attributed to either a real effect or to an experimental artefact. Tantra et al.26 suggested that the shift in ξ at low particle concentration of multi-walled carbon nanotubes, silica (LUDOX) and gold NPs, was due to an increase in the contribution of the signal from extraneous particulate matter. Small changes observed in zeta potential measurements at low particle concentrations indicate the adsorption of significant counterions from the solution on the particle surface. A possible counterion12 could be the OH− coming from the dissociation of water molecules, and the bicarbonate HCO3− and carbonate CO32− from the reactions, due to the presence of carbon dioxide (CO2) in solution from ambient gas. If the particle concentration is high, then the amount of adsorbed HCO3− can be neglected and the corresponding zeta potential becomes independent of the NP concentration.23 Therefore, the above results indicate that care must be taken in choosing appropriate particle concentration conditions for electrophoretic zeta potential measurements under standardised conditions of temperature and pH.
2.2. Physical properties
This subsection summarizes the most relevant physical properties for MeO NP characterisation for toxicity assessment.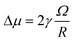 | (5) |
The chemical potential of an atom on a NP surface is higher in convex surfaces (i.e., with positive curvature) than in flat surfaces. Mass transfer from a flat surface to a convex one results in an increase of chemical potential, while the opposite occurs with concave surfaces.24
As previously mentioned, the μ of a NP dictates its shape, composition, and crystallographic structure. At the nanoscale, free energy and stress can induce changes in thermodynamic stability, modifying cell parameters27 and, as a consequence, causing structural transformations in the crystal structure of the NP with respect to the bulk.5 In other words, when mechanical and structural stabilities are balanced with free surface energy, then unstable crystalline bulk structures may become stable at the nanoscale. This phenomenon has been observed for MeO NPs such as TiO2,27 VOx,5 Al2O3,5 and MoOx.28
The ionic or covalent character of metal–oxygen bonding is related to the chemical potential of the NP. Ionicity can be affected by the NP size. The increase in ionic character is inversely proportional to the size of the particle21 and has direct effects on properties such as conductivity and chemical reactivity.29,30 In addition, the toxicity of NPs is strongly related to the electrostatic potential since NP–cell interaction mechanisms depend on electrostatic forces. Positive NPs are electrostatically attracted by negative bacterial membranes, where they can be absorbed (only this electrostatic type of interaction has been found experimentally).29
On the other hand, the conductivity of light is easily obtained by measuring the reflectivity and the absorption of solid materials. Reflectivity is a size-dependent property because it is affected by the size of particles and by the wave impedance of materials. As a result, nanoparticles are good candidates for developing high-performance optoelectronic devices such as semiconductor light-emitting diodes and laser diodes when the size is decreased as in the case of ZnO.35
| (αhν) = A(hν − Eg)n | (6) |
In the case of ZnO NPs, a broad band in absorption spectra is observed at around 369 nm, characteristic for pure ZnO. Bulk ZnO has a direct band gap of 3.34 eV.37 In the case of TiO2 NPs, the band gap is 3.15 eV.38
2.3. Chemical properties
Chemical reactivity properties are a key factor for the description of NP bioactivity and toxicity. Nanoparticle reactivity is determined by the chemical composition of the core and that of the surface and, if it exists (i.e., not being amorphous), by the crystallographic structure of the NM. The three key parameters responsible for the high reactivity of nanoparticles are the coordination environment of surface atoms, the redox properties, and the oxidation of the surface layers.Given their chemical properties, MeO NPs are widely used as absorbents and chemical catalysts.
Most ionic MeO NPs show a weak Brønsted activity to protonate bases. Nevertheless, SiO2, GeOx and BOx are exceptions to this rule40 due to their low valence, with the strongest Brønsted acidity appearing in oxides with valences of five or higher (WO3, MoO3, V2O5 and S-containing oxides).40
Finally, the isoelectric point (IEP) of NPs depends on the strength of the acid or base character of a material. In the context of toxicity, it has been reported that the IEP of TiO2 NPs affects their antibacterial activity.46
The solubility of a compound in a solvent at a given temperature is not only a thermodynamic property of the bulk but also depends on the dimension of the compound. Usually, the solubility of NPs is estimated by using a correction of Ostwald's equation,47 known as Freundlich's equation or Ostwald–Freundlich's equation:48
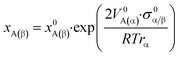 | (7) |
• Solubility is inversely proportional to size and independent of the surface area of the phase, following what Gibbs and Ostwald postulated.
• When size dependence on interfacial energy is taken under consideration, Ostwald's equation is correct.
• Numerical values from Ostwald's equation are similar to those from Ostwald–Freundlich's equation, showing that size dependence on solubility increases when dissolved particles have poorer “wettability” in the solution.
The use of in silico simulations provides an alternative approach to evaluate the solubility of MeO NPs. Recently, Escorihuela et al. have developed a method based on density functional tight binding models for evaluating qualitatively the solubility of NPs in aqueous solutions. This methodology was applied to ZnO NPs in water, although it can be easily transferred to other materials and solvents.50
For partially soluble MeO NPs, toxicity is also attributed to the release of free metal ions into the solution. Examples are ZnO and CuO,51 where toxicity cannot be satisfactorily explained only by the solubility of the NPs. Furthermore, the biological response can be modified depending on the kinetic effects of solubility; after 24 hours most of the metal ions and the aggregates are dissolved. That is why metal ion release is considered as one of the most important factors for toxicity assessment.52
ROS generation can be toxic both outside and inside cells. Metal ions released from NPs can enter cells and cause toxic effects such as oxidative stress, which impacts cell viability and may ultimately result in cell death. Extracellular ROS can also induce a series of oxidative stress reactions. For instance, the presence of OH radicals formed on MeO NPs due to ultraviolet (UV) radiation is a strong antibacterial mechanism26 present in a range of materials with a wide band gap such as ZnO.54
ROS generation is a problem not only in cytotoxicity but also in other fields of science. For example, MeO NPs can create defects in polymer electrolyte membranes (PEMs) used in fuel cells. The addition of non-stoichiometric ceria NPs induces effectively ROS generation during fuel cell operation, with the consequence of less operability duration and a degradation of the PEM.55
Experimental detection of ROS is done by using luminescent probes and electron spin resonance (ESR) spectroscopy. Since direct examination of the ability of NMs to generate ROS has very poor selectivity and poor photostability due to the short life of ROS, it is necessary to use spin trap molecules in both methods. For instance, dimethyl sulfoxide (DMSO) or 2′7′-dichloro-fluorescein diacetate (DCFH-DA) is used for ROS trapping. However, this method is not selective for different species since the scavengers do not selectively trap different ROS and it is difficult to distinguish, for example, the signal corresponding uniquely to OH radicals.45
A common problem in these two methods is that some MeO NPs, such as TiO2, have photocatalytic activity. Under illumination conditions, photocatalytic degradation of the spin trap may occur, giving a decrease in UV signal intensity against time. It is also difficult to obtain reproducible ROS spectra when nanoparticles do not form stable suspensions. In spite of this, ESR is a very stable and powerful method for the experimental characterisation of ROS.
3. Risk assessment
The risk assessment of bulk chemicals is usually performed as a three-step workflow including56 (1) exposure assessment (determining the exposure magnitude, frequency and duration in populations); (2a) hazard assessment (including hazard characterization, dose–response in organs, tissues, cells and toxicity mechanism); (2b) hazard identification (relevant properties producing the adverse effect); and (3) final risk quantification based on likelihood of exposure and hazard.Similarly, exposure and hazard are key factors that determine the risk that nanoparticles pose to the environment and humans. The physicochemical properties of MeO NPs contribute to their exposure and hazard profiles. Due to the large number of possible MeO NP combinations (e.g. size, shape, chemistry and surface modifications), performing a complete risk assessment for each nanoparticle would require significant time and resources.57 Despite the large diversity and complexity of the MeO nanoparticle, space detailed experimental studies including in vitro assays using bacteria cultures, in vivo experiments using rodents,58,59 and NP uptake mechanisms have started to provide data to elucidate relevant cytotoxicity mechanism. Burello and Worth60 proposed a theoretical model to predict the oxidative stress potential of oxide nanoparticles by comparing the redox potentials of relevant intracellular reactions with the energy structure of oxides. Horie et al.61 reviewed the cellular response of several manufactured NPs, giving special attention to MeO NPs. It was reported that MeO NPs induced an increase in the level of cell oxidation (ROS level).62 Comparing the results of experiments in human lung carcinoma A549 cells exposed to CuO, TiO2, ZnO, CuZnFe2O4, Fe3O4, and Fe2O3 NPs showed that the intracellular ROS level was highly increased in the cells exposed to CuO nanoparticles. The opposite effect was observed in cells exposed to CeO2 NPs in an oxidant stress test, induction of oxidative stress and antioxidative activity. These discrepancies are explained by the activation of antioxidative responses in cells or because the physicochemical characteristics of CeO2 NPs were different in each experiment; therefore, no standardisation in the characterisation activity of NPs evidences opposite results in assays.
Broadly speaking, toxicity (i.e., the potential of a substance to cause adverse health effects) is measured with respect to three parameters: dose, dimension and durability.15 In the case of NPs, the properties that induce specific toxicity effects and the mechanisms that mediate the adverse effects are still largely unclear.
As a consequence, many questions still remain open in terms of NP risk assessment. For instance, the UK government has requested advice from the Royal Society and the Royal Academy of Engineering to create a group of experts in nanoscience and nanotechnology. Similarly, in the USA, the National Nanotechnology Initiative was created to settle the innovations in this field. Worldwide institutions including the Organization for Economic Cooperation and Development (OECD), European Commission (REACH and Nanosafety Cluster), European Food Safety Authorisation, and Environmental Protection Agency also provide guidance for hazard assessment and risk evaluation of NPs.7 To date, the OECD has included in its Sponsorship Programme for Testing of Manufactured Nanomaterials a list of parameters, measurements, methods and endpoints which are considered as relevant for the regulation of NMs [ENV/JM/MONO(2006)19].18 Endpoints were categorized according to the state of dispersion, aggregation of NPs, size, surface area, porosity and surface reactivity. The document groups NPs into 11 categories of NMs and analyzes 24 different physicochemical test methods including physical identification, particle size distribution, shape, aspect ratios, agglomeration and aggregation, porosity, surface composition, crystal structure, and surface charge and reactivity. In addition to the above physicochemical properties, parameters for fate and exposure assessment such as zeta potential, water solubility and dustiness were also analysed. However, the only recommendations at this point are the use of harmonized methods and control of measurement conditions. In addition, it is necessary to develop guidance for developing NP descriptors, emphasizing the order of each descriptor and how to identify them in each technique, determining if the available information, even in the literature, is adequate and reliable.
In this context, computational (i.e., in silico) methods have emerged as an alternative for the evaluation of physicochemical properties of MeO NPs. In silico approaches provide the basic framework to implement intelligent testing strategies for hazard assessment of nanomaterials. The use of data-driven approaches to establish relationships between the structure of a nanoparticle and its physicochemical properties and bioactivity profile is an effective tool for the in silico hazard assessment.
4. Development of nano-QSARs and nano-QSPRs for MeO NPs
Methods based on structural similarity principles such as quantitative structure–property relationships (QSPRs) and quantitative structure–activity relationships (QSARs) are well-established and play a central role in the prediction of the properties and activity of bulk chemicals. The basic purpose of QSPR and QSAR is to correlate structural features (i.e., descriptors) with physicochemical properties or activity (e.g., toxicity), respectively. Descriptors can be developed from experimental measurements or calculated using computational chemistry methods. In general, the use of descriptors obtained via computational approaches is more effective than the use of experimental measurements since these calculations can be more easily extended to a much larger group of chemicals.When we extrapolate these methods to nanostructures, the terms nano-QSAR and QNAR (quantitative nanostructure–activity relationship) modelling are preferred.16 However, the extrapolation of these techniques to nanoscale materials is not straightforward. The main factors that hinder the development of nano-QSPRs and nano-QSARs are data scarcity, computational cost of developing descriptors representing the whole nanostructure, and the limited knowledge of the mechanisms that regulate nano-bio interactions. The most relevant features describing NPs include particle size, size distribution, crystal structure, shape, chemical composition, surface area and chemistry, and electronic properties.63
The properties of pristine nanoparticles (i.e., as synthesized) usually experience drastic changes and dynamic behaviour under exposure conditions (e.g., biological milieu). For instance, using the primary size of a nanoparticle as a predictor variable or as the basis to compute nanoparticle descriptors may be misleading or uninformative since the initial nanostructure of the pristine material could be completely different from the structure that emerges after exposure (e.g., formation of large aggregates and attachment of proteins to the NP surface). An additional confounding factor is that structurally similar nanoparticles may interact with biological systems through various mechanisms mediated by different biological receptors. As a consequence, completely different sets of properties and descriptors can be identified as the most predictive for nanoparticles with similar structure. All the above factors should be taken into account when developing nano-QSARs and nano-QSPRs.
4.1. Examples of nano-QSARs/QSPRs
This subsection provides a brief discussion of nano-QSPRs/QSARs developed using descriptors related to the properties discussed in section 2.One of the first attempts to develop a model was performed by Puzyn et al. using descriptors obtained from quantum chemistry calculations. The model was developed using a linear regression approach (eqn (8)) to predict in vitro toxicity for Escherichia coli bacteria:8
| log(EC50)−1 = 2.59 − 0.50ΔHMe+ | (8) |
Using a similar approach, Gajewicz et al. developed a nano-QSAR based on multilinear regression to predict the toxicity of metal oxides:63
| log(LC50)−1 = 2.47(±0.05) + 0.24(±0.05)ΔHfc + 0.39(±0.05)Xc | (9) |
Mikolajczyk et al.64 implemented a nano-QSPR model to predict the zeta potential using the correlation of two molecular descriptors: the spherical size, φ, and the weighted energy of the highest occupied molecular orbital (HOMO) for 15 MeO NPs:
| ζ = −11.26 − 4.46φ − 2.39εHOMO/nMe | (10) |
The model allowed one to quantitatively establish a relation between the zeta potential and the structural and electronic parameters of MeO NPs.
Recent development of new models for the simultaneous prediction of multiple toxicity endpoints gains importance as a comprehensive safety assessment, given the established toxicity of some NPs to both humans and the environment.65 The application of perturbation theory goes one step beyond traditional QSAR approaches. Kleandrova et al.66 used this tool on metal and metal oxide NPs. The aim was to make a QSAR model sensitive to modifications in NP compositions and experimental conditions. The descriptors used were molar volume, electronegativity, polarizability and NP size; in the case where a NM was formed by more than one element, these properties were normalized as the sum of the properties divided by the total number of atoms. Perturbation models aim to capture the sensitivity in the composition modification of the NPs versus experimental conditions and to determine if the variations in the structure and/or composition of NMs affect their toxicity. For these reasons, the original descriptors are modified by the moving average approach in order to create a new set of descriptors. Secondly, a set of pairs of NPs was created randomly wherein in each pair one particle was taken as a reference or initial state and the other particle was the predicted one. Perturbation theory was also applied by Luan et al.,67 together with quantitative structure–toxicity relationship (QSTR) in metal and metal oxide NPs, to predict different cytotoxicity profiles considering changes in sizes and measurement conditions.
A more detailed review of existing nano-QSPR and nano-QSARs can be found elsewhere.68
5. Conclusions
The evaluation of the toxicological effects of new materials plays a key role in the safe development of new products for general applications in electronics, cosmetics, optical devices, etc. In particular, NMs have shown an important increase in their applications, though their toxicity is still an open issue and several international organizations are trying to define and standardize methods to assess and control their hazardous effects.Due to the large number of possible MeO NPs, an exhaustive experimental evaluation would take many years to complete and would require significant economic resources. In silico modelling of NMs is a clear alternative for obtaining, from computational chemistry calculations, descriptors that can be related to the properties and toxicity of NPs using QSAR, QSPR or QSTR methodologies, of which we have discussed a few examples here.
This review provides a detailed discussion on the physicochemical properties that are considered to be the most relevant factors in the toxicity of NPs. Chemical properties and surface-related properties are the most crucial effects to describe the toxicity of NPs. Of particular interest are properties such as surface charge and pH-related effects as well as the solubility of nanoparticles and the subsequent shedding of metal ions that result in ROS formation on the NP surface.
5.1. Future works and research directions
The development of nano-QSARs and nano-QSPRs relies heavily on the volume and quality of available data. Although in the past years significant resources have been devoted to data curation and standardization, the information available for nanoparticles is still scarce relative to the information available for bulk chemicals and small molecules. As a result, current hazard models for nanoparticles have a limited applicability domain which restricts their use in regulatory risk assessment. Current data scarcity could be addressed by developing new high-throughput and high-content screening platforms for the rapid characterization of large nanoparticle libraries at multiple exposure conditions. Also, efficient computational approaches suitable for calculating whole nanoparticle descriptors are also required to provide the necessary structural information for model development.Novel approaches to nano-QSAR/QSPR development are also needed. For instance, a specific area that requires further exploration is the development of probabilistic nano-QSAR/QSPR models based on probability density functions of descriptor values that match the size distribution of nanoparticle dispersions. Similarly, the integrated use of multiple modalities of data (e.g., image data, numeric data and text data), both for the material and for the biological entities, could potentially open the door to model performance improvement.
In addition to assessing model performance, uncertainty quantification (UQ) and uncertainty propagation techniques should be included into the data-driven modelling workflows. Implementing proper uncertainty characterization schemes will contribute to informed decision-making during nanoparticle risk assessment.
As the amount of available data increases, data-intensive approaches such as deep learning can contribute to provide better linking between the structure of a nanoparticle and its physicochemical properties and activity. One of the main advantages of deep learning is the ability to learn highly efficient data representations, making the process of feature selection unnecessary. In this context, the use of advanced techniques such as transfer learning should be explored as a potential way of leveraging existing data for bulk chemicals to bootstrap deep learning networks for nanotoxicity.
Regarding future directions for the toxicity evaluation, there is also an urgent necessity for the standardisation of the experimental tests to challenge and validate the results of modelling studies. It is necessary that this standardisation works together with computational and experimental methodologies to gain future insight into risk assessment and other challenges. The use of in silico methods and advanced machine learning techniques to obtain NP descriptors will provide in the future the next generation of basic tools to implement intelligent testing strategies for and to support the estimation of the toxicity risk assessment of nanoparticles and regulatory decision-making.
Conflicts of interest
There are no conflicts of interest for the authors to declare.Acknowledgements
This work was supported by the project NanoDesk (SOE1/P1/E0215). RR acknowledges the support provided by the Laboratory Directed Research and Development Program at Pacific Northwest National Laboratory, a multiprogram national laboratory operated by Battelle for the U.S. Department of Energy.Notes and references
- 'Plenty of room' revisited, Nat. Nanotechnol., 2009, 4, p. 781, Editorial Search PubMed.
- A. Kumar, NANO39C - Nanotechnology in Environmental Applications: The Global Market, bcc Research, 2015 Search PubMed.
- A. Gallegos, E. Burello and A. Worth, Review of Computational approaches for predicting the physicochemical and biological properties of nanoparticles, European Commission, 2009, ISSN: 1018-5593 Search PubMed.
- L. K. Adams, D. Y. Lyon and P. J. J. Alvarez, Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions, Water Res., 2006, 40, 3527–3532 CrossRef PubMed.
- J. A. Rodríguez and M. Fernández Garcia, Synthesis, properties, and applications of oxide nanomaterials, Wiley-Interscience, Hoboken, New Jersey, 2007 Search PubMed.
- A. Nel, T. Xia, L. Mädler and N. Li, Toxic Potential of Materials at the Nanolevel, Science, 2006, 311(5761), 622–627 CrossRef PubMed.
- A. Gajewicz, B. Rasulev, T. C. Dinadayalane, P. Urbaszek, T. Puzyn, D. Leszczynska and J. Leszczynski, Advancing risk assessment of engineered nanomaterials: Application of computational approaches, Adv. Drug Delivery Rev., 2012, 64, 1663–1693 CrossRef PubMed.
- T. Puzyn, B. Rasulev, A. Gajewicz, X. Hu, T. P. Dasari, A. Michalkova, H.-M. Hwang, A. Toropov, D. Leszczynska and J. Leszczynski, Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles, Nat. Nanotechnol., 2011, 6, 175–178 CrossRef PubMed.
- K. L. Dreher, Health and Environmental Impact of Nanotechnology: Toxicological Assessment of Manufactured Nanoparticles, Toxicol. Sci., 2004, 77, 3–5 CrossRef PubMed.
- European Comission, Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, Off. J. Eur. Communities: Legis., 2006, 396, 1–849 Search PubMed.
- A. Hunt and P. Watkiss, Literature review on climate change impacts on urban city centres, 2007, OECD JT0323764 Search PubMed.
- M. Riedikerr, Coordination and Collaboration in European Research towards Healthy and Safe Nanomaterials, J. Phys.: Conf. Ser., 2011, 304, 012001 CrossRef.
- A. E. Nel, W. J. Parak, W. C. W. Chan, T. Xia, M. C. Hersam, C. J. Brinker, J. I. Zink, K. E. Pinkerton, D. R. Baer and P. S. Weiss, Where are we heading in nanotechnology environmental health and safety and materials characterization?, ACS Nano, 2015, 9, 5627–5630 CrossRef PubMed.
- A. Gajewicz, N. Schaeublin, B. Rasulev, S. Hussain and D. Leszczynska, Towards understanding mechanisms governing cytotoxicity of metal oxides nanoparticles: Hints from nano-QSAR studies, T. Puzyn and J. Leszczynski, Nanotoxicology, 2015, 9, 313–325 CrossRef PubMed.
- H. Zhang, Z. Ji, T. Xia, H. Meng, C. Low-Kam, R. Liu, S. Pokhrel, S. Lin, X. Wang, Y. P. Liao, M. Wang, L. Li, R. Rallo, R. Damoiseaux, D. Telesca, L. Mädler, Y. Cohen, J. I. Zink and A. E. Nel, Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation, ACS Nano, 2012, 6, 4349–4368 CrossRef PubMed.
- D. Fourches, D. Pu, C. Tassa, R. Weissleder, S. Y. Shaw, R. J. Mumper and A. Tropsha, Quantitative nanostructure–activity relationship modeling, ACS Nano, 2010, 4, 5703–5712 CrossRef PubMed.
- C. Sayes and I. Ivanov, Comparative study of predictive computational models for nanoparticle-induced cytotoxicity, Risk Anal., 2010, 30, 1723–1734 CrossRef PubMed.
- OECD, Physical-chemical parameters: measurements and methods relevant for the regulation of nanomaterials, 2016, p. JT03389225 Search PubMed.
- A. T. S. Wee, C. H. Sow and C. W. Shong, Science at the Nanoscale: An Introductory Textbook, Pan Stanford Publishing, 2016 Search PubMed.
- J. Ying, T. Zhang and M. Tang, Metal oxide nanomaterial QNAR models: available structural descriptors and understanding of toxicity mechanisms, Nanomaterials, 2015, 5, 1620–1637 CrossRef PubMed.
- J. M. McHale, A. Auroux, A. J. Perrotta and A. Navrotsky, Surface energies and thermodynamic phase stability in nanocrystalline aluminas, Science, 1997, 277, 788–791 CrossRef.
- Y. Zhang, M. Yang, N. G. Portney, D. Cui, G. Budak, E. Ozbay, M. Ozkan and C. S. Ozkan, Zeta potential: a surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells, Biomed. Microdevices, 2008, 10, 321–328 CrossRef PubMed.
- N. Wang, C. Hsu, L. H. Zhu, S. J. Tseng and J. P. Hsu, Influence of metal oxide nanoparticles concentration on their zeta potential, J. Colloid Interface Sci., 2013, 407, 22–28 CrossRef PubMed.
- G. Cao, Nanostructures and Nanomaterials - Synthesis, Properties and Applications, Imperial College Press, London, 2004 Search PubMed.
- P. Leroy, C. Tournassat and M. Bizi, Influence of surface conductivity on the apparent zeta potential of TiO2 nanoparticles, J. Colloid Interface Sci., 2011, 356, 442–453 CrossRef PubMed.
- R. Tantra, P. Schulze and P. Quincey, Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility, Particuology, 2010, 8, 279–285 CrossRef.
- H. Zhang and J. F. Banfield, Thermodynamic analysis of phase stability of nanocrystalline titania, J. Mater. Chem., 1998, 8, 2073–2076 RSC.
- Z. Song, T. Cai, Z. Chang, G. Liu, J. A. Rodriguez and J. Hrbek, Molecular level study of the formation and the spread of MoO3 on Au (111) by scanning tunneling microscopy and X-ray photoelectron spectroscopy, J. Am. Chem. Soc., 2003, 125, 8059–8066 CrossRef PubMed.
- R. Hoffmann, Solids and surfaces : a chemist's view of bonding in extended structures, Wiley-VHC, New York, 1988 Search PubMed.
- T. A. Albright, J. K. Burdett and M.-H. Whangbo, Orbital interactions in Chemistry, John Wiley & Sons, New York (USA), 2013 Search PubMed.
- C. Q. Sun, T. P. Chen, B. K. Tay, S. Li, Y. B. Zhang, H. Huang, L. K. Pan, S. P. Lau and X. W. Sun, An extended 'quantum confinement' theory: surface-coordination imperfection modifies the entire band structure of a nanosolid, J. Phys. D: Appl. Phys., 2001, 34, 3470–3479 CrossRef.
- B. J. Scott, G. Wirnsberger and G. D. Stucky, Mesoporous and mesostructured materials for optical applications, Chem. Mater., 2001, 13, 3140–3150 CrossRef.
- J. M. Luttinger and W. Kohn, Motion of electrons and holes in perturbed periodic fields, Phys. Rev., 1955, 97, 869–883 CrossRef.
- Y. D. Glinka, S. H. Lin, L. P. Hwang, Y. T. Chen and N. H. Tolk, Size effect in self-trapped exciton photoluminescence from SiO2-based nanoscale materials, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 085421 CrossRef.
- J. W. Li, X. J. Liu, L. W. Yang, Z. F. Zhou, G. F. Xie, Y. Pan, X. H. Wang, J. Zhou, L. T. Li, L. Pan, Z. Sun and C. Q. Sun, Photoluminescence and photoabsorption blueshift of nanostructured ZnO: Skin-depth quantum trapping and electron-phonon coupling, Appl. Phys. Lett., 2009, 95, 031906 CrossRef.
- J. Tauc, R. Grigorovici and A. Vancu, Optical properties and electronic structure of amorphous germanium, Phys. Status Solidi, 1966, 15, 627–637 CrossRef.
- M. Kahouli, A. Barhoumi, A. Bouzid, A. Al-Hajry and S. Guermazi, Structural and optical properties of ZnO nanoparticles prepared by direct precipitation method, Superlattices Microstruct., 2015, 85, 7–23 CrossRef.
- M. M. Khan, S. a. Ansari, D. Pradhan, M. O. Ansari, D. H. Han, J. Lee and M. H. Cho, Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies, J. Mater. Chem. A, 2014, 2, 637–644 RSC.
- D. J. Buttrey, Book Review - Metal Oxides: Chemistry and Applications ed. J. L. G. Fierro, National Council for Scientific Research (CSIC), Madrid, Spain. CRC Press/Taylor & Francis Group: Boca Raton, FL. 2006. xxii + 783 pp. ISBN 0-8247-2371-6, J. Am. Chem. Soc., 2006, 128, 11315–11316 CrossRef.
- M. Fernández-garcia and J. A. Rodriguez, Metal Oxide Nanoparticles, Nanomaterials: Inorganic and Bioinorganic Perspectives, Wiley, 2008 Search PubMed.
- Y. M. Chiang, E. B. Lavik, I. Kosacki, H. L. Tuller and J. Y. Ying, Nonstoichiometry and electrical conductivity of nanocrystalline CeO2−x, J. Electroceram., 1997, 1, 7–14 CrossRef.
- M. E. Franke, T. J. Koplin and U. Simon, Metal and metal oxide nanoparticles in chemiresistors: does the nanoscale matter?, Small, 2006, 2, 301 CrossRef.
- H. Maekawa, R. Tanaka, T. Sato, Y. Fujimaki and T. Yamamura, Size-dependent ionic conductivity observed for ordered mesoporous alumina-LiI composite, Solid State Ionics, 2004, 175, 281–285 CrossRef.
- B. M. Reddy, Met. Oxides Chem. Appl., 2006, vol. 108, pp. 215–246 Search PubMed.
- G. Busca, The Surface Acidity and Basicity of Solid Oxides and Zeolites, Metal Oxides: Chemistry and Applications, CRC Press, Taylor & Francis, Boca Raton, 2005, pp. 215–246 Search PubMed.
- A. B. Djurisic, Y. H. Leung, A. M. C. Ng, X. Y. Xu, P. K. H. Lee, N. Degger and R. S. S. Wu, Toxicity of metal oxide nanoparticles: mechanisms, characterization, and avoiding experimental artefacts, Small, 2015, 11, 26–44 CrossRef PubMed.
- W. Ostwald, Z. Phys. Chem., Stoechiom. Verwandtschaftsl., 1900, 34, 495–503 Search PubMed.
- H. Freundlich and E. Makelt, Z. Elektrochem. Angew. Phys. Chem., 1909, 15, 161–165 Search PubMed.
- G. Kaptay, On the size and shape dependence of the solubility of nano-particles in solutions, Int. J. Pharm., 2012, 430, 253–257 CrossRef PubMed.
- L. Escorihuela, A. Fernández, R. Rallo and B. Martorelll, Molecular dynamics simulations of zinc oxide solubility: From bulk down to nanoparticles, Food Chem. Toxicol., 2018, 112, 518–525 CrossRef PubMed.
- R. L. M. Robinson, M. T. D. Cronin, A.-N. Richarz and R. Rallo, An ISA-TAB-Nano based data collection framework to support data-driven modelling of nanotoxicology, Beilstein J. Nanotechnol., 2015, 6, 1978–1999 CrossRef PubMed.
- M. Horie, K. Fujita, H. Kato, S. Endoh, K. Nishio, L. K. Komaba, A. Nakamura, A. Miyauchi, S. Kinugasa, Y. Hagihara, E. Niki, Y. Yoshida and H. Iwahashi, Association of the physical and chemical properties and the cytotoxicity of metal oxide nanoparticles: metal ion release, adsorption ability and specific surface area, Metallomics, 2012, 4, 350–360 RSC.
- N. E. Schultz, Y. Zhao and D. G. Truhlar, Density Functionals for Inorganometallic and Organometallic Chemistry, J. Phys. Chem. A, 2005, 109, 11127–11143 CrossRef PubMed.
- A. Lipovsky, Z. Tzitrinovich, H. Friedmann, G. Applerot, A. Gedanken and R. Lubart, EPR study of visible light-induced ROS generation by nanoparticles of ZnO, J. Phys. Chem. C, 2009, 113, 15997–16001 CrossRef.
- P. Trogadas, J. Parrondo and V. Ramani, CeO2 surface oxygen vacancy concentration governs in situ free radical scavenging efficacy in polymer electrolytes, ACS Appl. Mater. Interfaces, 2012, 4, 5098–5102 CrossRef PubMed.
- A. P. Worth, The role of QSAR methodology in the regulatory assessment of chemicals, Recent Adv. Qsar Stud. Methods Appl., 2010, vol. 8, pp. 367–382 Search PubMed.
- S. Dekkers, A. G. Oomen, E. A. J. Bleeker, R. J. Vandebriel, C. Micheletti, J. Cabellos, G. Janer, N. Fuentes, S. Vázquez-Campos, T. Borges, M. J. Silva, A. Prina-Mello, D. Movia, F. Nesslany, A. R. Ribeiro, P. E. Leite, M. Groenewold, F. R. Cassee, A. J. A. M. Sips, A. Dijkzeul, T. van Teunenbroek and S. W. P. Wijnhoven, Towards a nanospecific approach for risk assessment, Regul. Toxicol. Pharmacol., 2016, 80, 46–59 CrossRef PubMed.
- H.-W. Chen, S.-F. Su, C.-T. Chien, W.-H. Lin, S.-L. Yu, C.-C. Chou, J. J. W. Chen and P.-C. Yang, Titanium dioxide nanoparticles induce emphysema-like lung injury in mice, FASEB J., 2006, 20, 2393–2395 CrossRef PubMed.
- P. V. Asharani, Y. L. Wu, Z. Gong and S. Valiyaveettil, Toxicity of silver nanoparticles in zebrafish models, Nanotechnology, 2008, 19, 255102 CrossRef PubMed.
- E. Burello and A. P. Worth, A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles, Nanotoxicology, 2011, 5, 228–235 CrossRef PubMed.
- M. Horie, H. Kato, K. Fujita, S. Endoh and H. Iwahashi, In vitro evaluation of cellular response induced by manufactured nanoparticles, Chem. Res. Toxicol., 2012, 25, 605–619 Search PubMed.
- H. Zhang, Z. Ji, T. Xia, H. Meng, C. Low-Kam, R. Liu, S. Pokhrel, S. Lin, X. Wang, Y.-P. Liao, M. Wang, L. Li, R. Rallo, R. Damoiseaux, D. Telesca, L. Maedler, Y. Cohen, J. I. Zink and A. E. Nel, Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation, ACS Nano, 2012, 6, 4349–4368 CrossRef PubMed.
- A. Gajewicz, N. Schaeublin, B. Rasulev, S. Hussain, D. Leszczynska, T. Puzyn and J. Leszczynski, Towards understanding mechanisms governing cytotoxicity of metal oxides nanoparticles: hints from nano-QSAR studies, Nanotoxicology, 2015, 9, 313–325 CrossRef PubMed.
- A. Mikolajczyk, A. Gajewicz, B. Rasulev, N. Schaeublin, E. Maurer-Gardner, S. Hussain, J. Leszczynski and T. Puzyn, Zeta potential for metal oxide nanoparticles: a predictive model developed by a nano-quantitative structure–property relationship approach, Chem. Mater., 2015, 27, 2400–2407 CrossRef.
- N. Basant and S. Gupta, Multi-target QSTR modeling for simultaneous prediction of multiple toxicity endpoints of nano-metal oxides, Nanotoxicology, 2017, 11, 339–350 CrossRef PubMed.
- V. V. Kleandrova, F. Luan, H. Gonzalez-Diaz, J. M. Ruso, A. Melo, A. Speck-Planche and M. N. D. S. Cordeiro, Computational ecotoxicology: Simultaneous prediction of ecotoxic effects of nanoparticles under different experimental conditions, Environ. Int., 2014, 73, 288–294 CrossRef PubMed.
- F. Luan, V. V. Kleandrova, H. González-Díaz, J. M. Ruso, A. Melo, A. Speck-Planche and M. N. D. S. Cordeiro, Computer-aided nanotoxicology: assessing cytotoxicity of nanoparticles under diverse experimental conditions by using a novel QSTR-perturbation approach, Nanoscale, 2014, 6, 10623–10630 RSC.
- G. Chen, M. Vijver, Y. Xiao and W. Peijnenburg, A Review of Recent Advances towards the Development of (Quantitative) Structure-Activity Relationships for Metallic Nanomaterials, Materials, 2017, 10, 1013 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |
