New aspects of the environmental risks of quantum dots: prophage activation†
Juan
Xu‡
a,
Huan
He‡
 a,
Ying-Ying
Wang
a,
Ren
Yan
a,
Lian-Jiao
Zhou
a,
Yu-zhu
Liu
a,
Feng-Lei
Jiang
a,
Ying-Ying
Wang
a,
Ren
Yan
a,
Lian-Jiao
Zhou
a,
Yu-zhu
Liu
a,
Feng-Lei
Jiang
 *a,
Thomas
Maskow
*a,
Thomas
Maskow
 *b and
Yi
Liu
*acd
*b and
Yi
Liu
*acd
aState Key Laboratory of Virology & Key Laboratory of Analytical Chemistry for Biology and Medicine (MOE), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430072, P. R. China. E-mail: fljiang@whu.edu.cn; yiliuchem@whu.edu.cn; Fax: +86 27 68754067; Tel: +86 27 68756667
bUFZ, Helmholtz Centre for Environmental Research, Department of Environmental Microbiology, Permoserstr. 15, 04318 Leipzig, Germany. E-mail: thomas.maskow@ufz.de
cKey Laboratory of Coal Conversion and Carbon Materials of Hubei Province, College of Chemistry and Chemical Engineering, Wuhan University of Science and Technology, Wuhan 430081, P. R. China
dCollege of Chemistry and Material Sciences, Guangxi Teachers Education University, Nanning 530001, P. R. China
First published on 28th May 2018
Abstract
A few thousand tons of nanoparticles and quantum dots (QDs) are produced yearly worldwide, and a significant amount is released into ecosystems. This knowledge has stimulated numerous studies on the toxicological properties of these nanomaterials. However, an important ecotoxicological aspect has been largely overlooked: the activation of silent viruses in bacteria (the so-called prophages). This is particularly important because, once the prophages are activated, phage replication using bacterial hosts is an autocatalytic process with a potentially exponential rate of bacteria killing under certain conditions. To shed light on these underestimated processes, the interactions of differently functionalized CdTe QDs with E. coli containing lambda prophages were investigated. We found that prophages can be activated with as little as a nanomolar concentration range of QDs. DNA damage due to oxidative stress induced by the CdTe QDs was revealed as the main reason for the prophage activation. The contribution of freely dissociated Cd2+ to prophage activation was on the order of 15 to 25%. Our pioneering work is intended to provide the first examination to better understand the role of nanoparticles in aquatic ecosystems.
Environmental significanceQuantum dots are widely used, and their interactions with biological/environmental systems are inevitable. Their biosafety needs to be fully considered and investigated. However, the potential influence of QDs on ecosystems through the activation of silent viruses inside bacteria is mostly neglected. Once prophages are activated, phages will be released into the environment. These phages can infect other bacteria, which might lead to significant destruction of the micro-ecological balance. In our work, differently coated CdTe QDs were used to investigate their interactions with E. coli containing prophages. We found that prophages can be activated with as little as a nanomolar concentration range of QDs. |
Introduction
Nanoparticles, especially quantum dots (QDs), have numerous applications in various areas such as drug delivery, biosensors, biomedical imaging, solar energy generation and electronic devices due to their desirable optical and electrochemical properties.1 The yearly production of nanomaterials is estimated to be 5000 t (TiO2), 500 t (Ag) and 350 t (carbon nanotubes).2 Recent investigations have raised concerns over adverse effects on ecosystem health after the release of nanomaterials into soil and water3 because QDs attack bacteria, fungi, algae and protozoa on different organismic levels even at very low concentrations.4 Additionally, QDs can be enriched by surface adsorption, bio-accumulation and biomagnification in aquatic ecosystems.5 Numerous studies have revealed the adverse effect of QDs on bacterial growth and biodegradation processes6–8 and have defined the toxicity targets.9,10 However, the potential influence of QDs on ecosystems through the activation of silent viruses inside bacteria is mostly neglected. When temperate bacteriophages infect bacteria, they integrate their genome into the chromosome(s) of their host and replicate their DNA along with the bacterial chromosome without producing viruses.11 This phenomenon is called lysogeny, and the integrated phage genome is called the prophage. Evidence has shown that lysogenic bacteria are common in water and soil environments,12 and the proportion of bacterial strains containing prophages may be as high as 80%.13 Normally, lysogenic bacteria could be spontaneously activated at a low speed even in the absence of exogenic stress. Environmental factors such as UV irradiation, chemical exposure and temperature alteration can activate the prophages.14 A common feature of prophage activation is damage to the bacterial DNA and a subsequent irreversible shift of lysogeny to lysis.15 Numerous results have also pointed out that the DNA breaks are critical for the switch of lysogenic bacteria into the lytic cycle.16 As a result, progeny phages capable of infecting other bacteria are spewed into the environment. This could possibly cause a significant reduction in the abundance of host species and community composition, and finally result in the destruction of the micro-ecological balance.17 Several conventional chemicals (organic and inorganic)18–20 have been reported to activate prophages. In light of this evidence, and considering the ecological importance of prophages, it is surprising that the activation of prophages by nanomaterials and QDs has not been reported. There is evidence suggesting that the uptake of QDs is accompanied by the release of heavy metal ions and the production of reactive oxygen species (ROS).6,7,21 ROS are known to damage DNA and thereby activate prophages. The ROS production rate can even be photochemically accelerated using nanocrystals and light.22,23 Photochemically activated nanocrystalline TiO2 is already reported to induce prophages.24 Additionally, evidence has shown that heavy metal ions like Cd2+, Cu2+ and Ag+ cause damage to the bacteria DNA.25,26 It is important to clarify these findings to prove whether QDs alone (without light) can activate prophages and to determine the critical threshold concentrations. Furthermore, the possible mechanisms should be illuminated. Finally, the influence of the chemical structure of QDs on prophage activation has to be considered because the biological activities of QDs are closely related to their chemical structures.27 We suggest E. coli DSM4230 with λ prophages and CdTe with different surface ligands as suitable objects of investigation because there is already a large body of knowledge about both systems.Materials and methods
Materials
CdCl2 (99.99%), 3-mercaptopropionic acid (MPA, 99%), L-glutathione (GSH, reduced, 98%), N-acetyl-L-cysteine (NAC), DL-dithiothreitol (DTT), L-ascorbic acid (Vc), 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and tellurium powder (99.999%, approximately 200 mesh) were obtained from Sigma-Aldrich and were used without further purification. All other reagents were of analytical grade. Ultrapure water with a resistivity of 18.2 MΩ cm−1 (Millipore Simplicity) was used in all aqueous solutions.Preparation and quantification of CdTe QDs
CdTe QDs capped with MPA and GSH were synthesized according to Xiang et al.27 Before using them, freshly synthesized QDs were washed three times with 2-propanol. Afterwards, the QDs were dispersed in ultrapure water, and the solutions were dialyzed for 4 h to remove impurities. The resultant QDs were stored at 4 °C in the dark for the subsequent experiments. The QDs were quantified by UV/VIS spectroscopy using the extinction coefficients at 524 nm (MPA–CdTe QDs) and 504 nm (GSH–CdTe QDs).28Bacterial strains and culture conditions
E. coli DSM4230 (obtained from DSMZ Braunschweig, Germany) (without (λ−) and with prophages (λ+)) was used. The prophage was established in the λ+ strain as described by Xu et al.29 In short, E. coli DSM 4230 was activated by overnight cultivation in LB medium to a final OD = 0.36 (corresponding to 2 × 108 CFU mL−1). 1 mL of this E. coli suspension was infected with the lambda phage (DSM 4499) (with a final titer of 5 × 107 PFU mL−1) corresponding to a MOI (multiplicity of infection) of 0.25. The mixture was incubated for 30 min at 37 °C without shaking, serially diluted and plated on LB agar. After 2 days of incubation at 37 °C and further incubation at room temperature, single colonies inside the phage plaques were isolated. The isolates were purified several times, checked for lambda phage segregation and sensitivity, and used for further investigations. All growth experiments were conducted at 37 °C. Cultures were maintained on LB medium amended with maltose (2 g L−1) and MgSO4·7H2O (0.12 g L−1). All media were autoclaved at 121 °C; the final pH was 7.2 ± 0.2, and the buffer capacity was high enough to maintain the pH for 20 minutes. The various amounts of QDs were aseptically added to the medium. The final pH was 7.2 ± 0.2, and the buffer capacity was high enough to maintain the pH during growth.Quantification of bacterial growth and phage propagation
Bacterial growth influenced by phage propagation was quantified off-line (as the optical density at 600 nm) and on-line (as the metabolic heat by microcalorimetry) in independent experiments. The optical densities were determined with a Hitachi U-2900 UV-vis spectrophotometer (Hitachi High-Tech, Tokyo, Japan). Growth was monitored on-line using a thermal activity monitor III (TAM III, TA Instruments, New Castle, USA). The ampoules and caps were autoclaved (30 min, 121 °C) before the experiment. Electric gain calibrations were regularly performed. Next, 1 mL of LB agar was put into the ampoules, and 10 μL of the bacterial suspension (1.9 mL of LB medium, 0.09 mL of E. coli (λ−), 0.01 mL of E. coli (λ+); OD = 0.1 each in LB medium) with QDs was dropped onto the solid agar. The ampoules were closed and made airtight before calorimetric monitoring.Quantification of the phage
The concentrations of induced phages were determined by the two-layer method.30 In this technique, a phage particle causes a plaque in a bacterial layer. The results are provided in PFU (plaque forming units). The basic layer contained LB medium in 1.4% agar, and the cover layer contained 0.7% agar and the exponentially growing indicator bacteria (E. coli (λ−); OD = 0.3). Next, 1 mL of the sample for PFU quantification was mixed with 100 μL of chloroform. The samples were centrifuged with 4500g for 20 minutes at 4 °C. The chloroform-free supernatants were stored in a refrigerator at 4 °C before the final PFU quantification, in which 10 μL serial dilutions of the cell-free supernatants were dropped onto the double-layer plates. The plates were incubated overnight at 37 °C, and the plaques were counted on the following day as the PFU in each drop area.Determination of the intracellular ROS level
E. coli (λ+) strains cultured overnight were harvested, washed twice with PBS and resuspended in tubes with PBS. Then, the microorganisms were cultivated in PBS in the presence of different concentrations of QDs. Finally, the cells were incubated with PBS buffer containing 2′,7′-dichlorofluorescein diacetate (DCFH-DA, 5 μM) for 30 min in the dark at 37 °C. The ROS level was flow cytometrically analyzed (C6 flow cytometer, BD Biosciences, USA).Quantification of superoxide dismutase activity and of lipid peroxides
E. coli (λ+) strains were cultivated under the same conditions as in the growth experiments. Afterwards, E. coli (λ+) was washed twice with PBS and disrupted using an ultrasonic cell disruption system. The activity of the superoxide dismutase (SOD) was assayed using a water-soluble tetrazolium salt (WST-8) kit (total superoxide dismutase assay kit with WST-8, Beyotime).31 The products of the lipid peroxidation (malondialdehyde, MDA) were quantified by the thiobarbituric reacting substances (TBARS) assay. A lipid peroxidation MDA assay kit (Beyotime, Nantong, China) was used as described by Liu et al.32Protective agents
To protect the bacteria against oxidative stress, N-acetyl-L-cysteine (NAC 2 mM), GSH (2 mM), dithiothreitol (DTT 1 mM), and vitamin C (Vc 1 mM) were applied.Toxicity of Cd2+
An attempt to reduce the toxicity of the heavy metal ion Cd2+ was made by masking the ion with ethylene diamine tetraacetic acid (EDTA, 1 mM). To demonstrate the success of masking, the concentration of free Cd2+ was determined with and without EDTA. For this purpose, the CdTe QDs samples with and without EDTA were dialyzed in deionized water using a 200 Da membrane for 2 days. The solution outside of the dialysis tube was reduced in volume to 1 mL. The concentrate was mixed with concentrated nitric acid in an ampule and evaporated to dryness. Finally, 8 mL of 3% nitric acid was used to wash the ampule, and the solution was prepared for the analysis using inductively coupled plasma atomic emission spectroscopy (ICP-AES).Results
Synthesis and characterization of CdTe QDs
CdTe QDs with two different surface ligands were prepared in an aqueous medium as described by Xiang et al.27 Transmission electron microscopy (TEM) was used to measure the morphology and size distribution of the CdTe QDs. As depicted in Fig. 1, CdTe QDs with a ligand independently exhibit a spherical shape and do not agglomerate. The average diameter of the CdTe QDs coated with MPA and GSH were determined using the microscopy data and were found to be 2.3 ± 0.5 nm and 2.2 ± 0.2 nm, respectively. The UV-visible absorption (red) and photoluminescence (green) spectra of the QDs at room temperature are shown in Fig. 1B and D. MPA–CdTe QDs and GSH–CdTe QDs exhibit a well-resolved first electronic transition absorption maximum at 524 and 504 nm, indicating a narrow CdTe QD size distribution. The size distribution of the QDs determines the width of the peaks.21 To characterize the potential electrostatic interactions of the QDs and the E. coli surface, the zeta potential of MPA–CdTe and GSH–CdTe QDs was quantified to be −22.3 mV and −24.0 mV, indicating a negatively charged surface for the QDs.Activation of prophages by CdTe QDs
In our previous work, a method of microcalorimetry for the monitoring of prophage activating chemicals was developed.29 The method is based on the difference between the metabolic heat production rates of bacteria with prophages (λ+) and without prophages (λ−) under the influence of prophage activating chemicals. Later research revealed (proved by simulations and experiments33) that a mixture of λ+ and λ− as a bioindicator is more sensitive against the chemical activation than the pure λ+ strain alone.34 The optimum ratio of λ−/λ+ is 9/1. This is additionally confirmed by experiments shown in the ESI† (Fig. S1†). Here, different mixtures of λ+ and λ− strains were exposed to the same concentration of a prophage-activating chemical (mitomycin C). To monitor the prophage-activating properties of QDs, E. coli (λ−) was mixed with E. coli (λ+) at the optimum ratio of 9/1 (cell number). To be able to analyze the influence of MPA–CdTe QDs on prophage activation, growth curves from the described bacterial mixture and the E. coli (λ−) were monitored from the optical density at 600 nm (ref. 35) (Fig. 2). As shown in Fig. 2A, the inhibitive effects of the MPA–CdTe QDs on E. coli (λ−) at low concentrations (<1 nM) were negligible. Differently, the inhibition efficiency of MPA–CdTe QDs in the bacterial mix was much stronger (Fig. 2B). At a concentration of 10 nM, the growth of the mixed culture was completely suppressed. The reasons for the different behavior for the mixed culture were the activation of the lambda prophages, the subsequent production of phages, and the final infection of further bacteria, as shown from the formation of phage plaques (Fig. 2C). The amount of phages depended on the concentration of the CdTe QDs (Fig. 2D). Notably, MPA–CdTe QDs already activate lambda prophages at a concentration of 0.5 nM, which is not toxic to E. coli (λ−). Both the MPA–CdTe and GSH–CdTe QDs could activate lambda prophages. However, the MPA–CdTe QDs were more effective than GSH–CdTe QDs (see Fig. S2 in the ESI†). To exclude potential distortions by sampling and to prevent biases, the prophage-activating properties of the CdTe QDs were additionally monitored by microcalorimetry in real time (Fig. 3). Microcalorimetry has been proved to be a simple method to analyze the influence of chemicals on silent prophages by monitoring the growth processes in real time.36 Moreover, the monitoring of the final concentration of free phages is a less suited indicator of prophage activation due to the very rapid adhesion of phages to bacteria, especially under the conditions used (in the presence of maltose and magnesium).37E. coli without prophages (λ−) and the E. coli mixtures (λ−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λ+ = 9
λ+ = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were grown in the presence of different concentrations of CdTe QDs on the surface of LB agar to ensure that the culture always had enough oxygen for respiratory metabolism38 (Fig. 3). The on-line method confirms the results of the off-line measurements. It should be noted that the concentrations of the QDs used in microcalorimetry were higher than those used in the off-line method. This was necessary because the exchange absorption rates for QDs on the agar surface were relatively lower than in LB medium. The transport of QDs plays a role in the growth on solid surfaces. Similar to the off-line experiments, in the microcalorimetry experiments, the number of phages increased in a dose-dependent manner (Fig. 3, inserts).
1) were grown in the presence of different concentrations of CdTe QDs on the surface of LB agar to ensure that the culture always had enough oxygen for respiratory metabolism38 (Fig. 3). The on-line method confirms the results of the off-line measurements. It should be noted that the concentrations of the QDs used in microcalorimetry were higher than those used in the off-line method. This was necessary because the exchange absorption rates for QDs on the agar surface were relatively lower than in LB medium. The transport of QDs plays a role in the growth on solid surfaces. Similar to the off-line experiments, in the microcalorimetry experiments, the number of phages increased in a dose-dependent manner (Fig. 3, inserts).
Oxidative stress induced by CdTe QDs
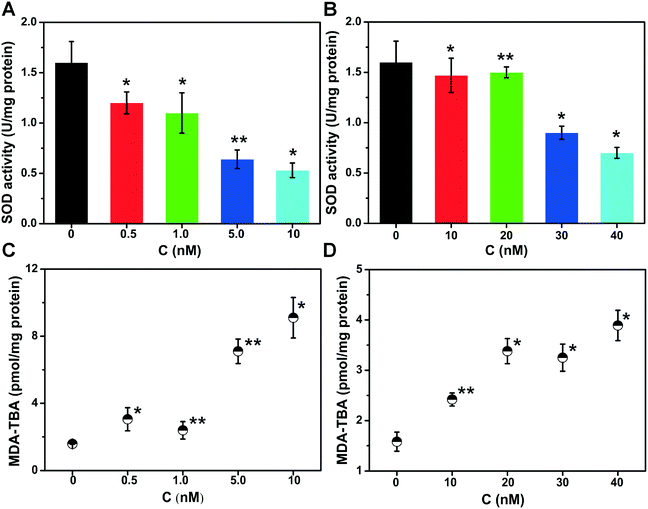
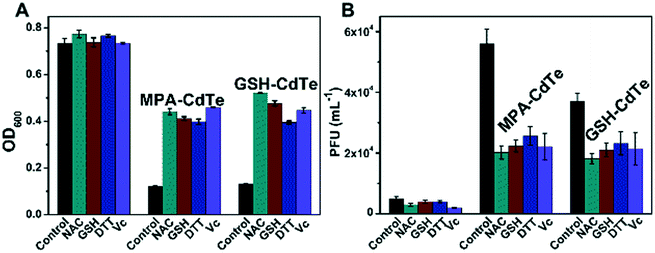
It has been well established that QDs affect organisms mainly through the generation of ROS and the release of Cd2+.39 ROS are known to attack DNA.40 For these reasons, the levels of ROS inside the bacterial cells were investigated flow cytometrically using the fluorescent probe DCFH-DA. DCFH-DA is taken up by cells and hydrolyzed by cellular esterase to yield DCFH, which is oxidized by ROS to the fluorescent stain DCF.41 Thus, the fluorescence intensity reflects the ROS level within the cells. The increase in the fluorescence intensity after treatment with MPA–CdTe QDs or GSH–CdTe QDs indicates a significant increase in the cellular ROS level (Fig. 4), supporting the assumption that an oxidative attack on DNA occurs. Additionally, the effect of MPA–CdTe QDs being stronger than that of GSH–CdTe QDs is supported by the ROS measurement. The difference in the oxidative damage induced by the two types of CdTe QDs could also be caused by the activities of antioxidative enzymes.42 Thus, the activity of antioxidative enzymes may be an important indicator for evaluating cellular oxidative stress.43 In this work, the activity of superoxide dismutase (SOD), one of the most crucial enzymes in the ROS elimination system, was monitored. In a reaction catalyzed by SOD, two molecules of superoxide form hydrogen peroxide and molecular oxygen.43 However, if the amount of oxygen free radicals exceeds the clearance capacity of SOD, ROS will react with amino acid sulfur- (or selenium), which will decrease the activity of SOD.44,45 Polyunsaturated fatty acids will be attacked, because of their multiple double bonds, which make them excellent targets for free radical attacks.46 Both types of CdTe QDs reduced the SOD activity in a dose-dependent manner (Fig. 5). Again, the effect of MPA–CdTe QDs is much stronger than the effect of GSH–CdTe QDs. Oxidative damage to lipid molecules has also been regarded as an indicator of the occurrence of oxidative stress in cells.47 Malondialdehyde48 (MDA), a natural product of the lipid oxidation of organisms, was used to quantitatively determine the level of oxidative damage to lipids in lysogenic E. coli. Fig. 5C and D demonstrate a dose-dependent relationship between MDA and CdTe QD concentrations, suggesting the occurrence of significant oxidative damage of the lipids. Again, the lipid damage is greater for treatment with MPA–CdTe QDs than with GSH–CdTe QDs. To investigate whether oxidative stress is actually responsible for the activation of phages by CdTe QDs, a series of general antioxidants (N-acetyl-L-cysteine: NAC, L-glutathione: GSH, DL-dithiothreitol: DTT and L-ascorbic acid: VC)49 were applied. The fluorescence intensity after the addition of the antioxidants (Fig. S3 in the ESI†) demonstrates effective elimination of the ROS produced by the CdTe QDs. Notably, the number of phages decreased by approximately 65% and 45% for the two CdTe QDs after the addition of antioxidants, indicating effective suppression of the prophage induction activity (Fig. 6). Consequently, the survival rates for the bacteria increased. | ||
| Fig. 4 Dosage dependence of the production of reactive oxygen species (ROS) for MPA–CdTe QDs (A) and GSH–CdTe QDs (B), *: P < 0.05, **: P < 0.01. | ||
Role of the CdTe QD dissociation
Cd2+, a dissociation product of CdTe QDs, is a toxic compound and could be responsible for the observed lambda prophage activation. Fig. 7 depicts the effects of Cd2+ and EDTA-masked Cd2+ on the bacterial growth and phage production. Interestingly, free Cd2+ (≥3 μM) activated lambda prophages, but it was almost nontoxic to E. coli (λ−). This indicates that the release of Cd2+ may also contribute to the prophage induction activity of CdTe QDs. To estimate the contribution of free Cd2+ under physiological conditions, mixtures of E. coli (λ+ and λ−) were simultaneously incubated with CdTe QDs and EDTA. EDTA (a popular chelating agent for Cd2+) was chosen to mask the heavy metal ion. Indeed, the concentration of free Cd2+ was drastically reduced after incubation with EDTA, as shown in Table 1. The number of phages decreased by 15% to 25%, indicating the potential contribution of Cd2+. Notably, the protective effect of EDTA for GSH–CdTe QDs is stronger than for MPA–CdTe QDs. This might be attributed to the dosage of GSH–CdTe QDs being four times higher than that of MPA–CdTe QDs which increased the concentration of free Cd2+.| Concentration of Cd2+ | Without EDTA (μM) | With EDTA (μM) |
|---|---|---|
| MPA–CdTe QDs | 2.12 ± 0.07 | 0.24 ± 0.04 |
| GSH–CdTe QDs | 5.75 ± 0.06 | 0.21 ± 0.03 |
Obviously, EDTA is also able to form metal complexes with a component of the medium (i.e. Mg2+), which could potentially affect the phage infection. To explore such potential side effects, EDTA-masking experiments in a medium without Mg2+ were performed. The results shown in Fig. S4† demonstrate that the effect of Mg2+ reduction was not significant. We can find that the protective effects of EDTA were similar in magnesium-containing and magnesium-free media, indicating that the protective effects of EDTA are not related to the binding of Mg2+.
Discussion
The toxicity of nanomaterials and in particular of QDs has become a hot topic in recent years due to their accelerated development and growing applications in various fields. Here, a new aspect of the ecotoxicology of such materials is illuminated. To the best of our knowledge, this is the first report concerning the activation of silent viruses inside bacteria (the so-called prophages) by QDs. These viruses, once released, may infect and kill further bacteria and therefore disturb ecosystem functions.For the investigation, spherical CdTe QDs were synthesized by the aqueous synthesis method using the coating ligands MPA and GSH. The sizes of the QDs were 2.3 ± 0.5 nm (MPA–CdTe QDs) and 2.2 ± 0.2 (GSH–CdTe QDs), which are in the typical size range exhibiting useful optical properties.50 Because the direct measurement of the charge of the surface of QDs and bacteria is difficult,51 we used the zeta potential to characterize the electrostatic interactions between QDs and E. coli. The zeta potential of the QDs is negative (MPA–CdTe: −22.3 mV, GSH–CdTe QDs: −24.0 mV) as is the zeta potential of E. coli (between −34 and −48 mV)52 in opposition to the electrostatic attraction. The activation of the lambda prophage by the different CdTe QDs is demonstrated by a reduction in the growth rate (due to killing of the bacteria) as well as by an increase in the active phage number. This finding is confirmed by on-line measurements of the metabolic heat. The strength of the lambda prophage activating properties of the QDs depends on their chemical structure, as demonstrated by the examples of differently coated CdTe QDs (with MPA and GSH). The activation capability of MPA–CdTe QDs is stronger than that of GSH–CdTe QDs. This result is consistent with the previous finding that the biological impacts of QDs were closely related to their specific physicochemical properties including the size, surface charge, surface modification and core/shell materials.53,54
It is widely accepted that changes in DNA (structural or fragmentation) are regarded as a common and essential event for the transition to the lytic cycle of phage replication. Treatments with DNA-damaging agents, leading to an SOS response within the bacteria, cause the activation of the RecA protein, inducing the expression of the phage genome. The observation of activated phages in CdTe QD treated lysogenic bacteria indicated that the chromosomal DNA of the bacteria has been damaged. The most likely reason for the DNA damage is oxidative stress, as demonstrated by the quantification of the ROS level and the protective effects of the ROS scavengers. It has been well established that an increase in DNA damage is an inevitable consequence of raised ROS levels.55 This theory is further supported by the reduction in the superoxide dismutase activity in a dose-dependent manner. This is fatal because not only does the amount of ROS increases but also the ability of the cells to resist decreases. The final proof of the mechanism of CdTe QDs was the formation of oxidation products such as lipid peroxides. The oxidative stress has been reported for several other nanomaterials including metal nanoparticles,56 metal oxide nanoparticles,57 carbonaceous nanomaterials58 and nanopolymers.59
It has long been known that exposure of cells to Cd2+ induces DNA damage, e.g., DNA single- and double-strand breaks and DNA–protein crosslinks.25,26 Thus, the phage-activating properties can be caused by the CdTe QDs themselves in addition to the dissociation product of Cd2+. The results of the growth experiments with Cd2+ show obvious prophage activation caused by the heavy metal ion. Experiments with CdTe QDs in the presence of a chelating agent allow the estimation of the contribution of dissociated Cd2+ (between 15 and 25% of the total effect).
In summary, our study suggests that more caution must be used with regard to the ecotoxicological properties of nanomaterials. The CdTe QD concentration needed to activate prophages is far less than the concentration needed to inhibit bacterial growth. More importantly, during the test measurements, the bacteria were only exposed to the nanomaterial for 8 h. Long-time exposure may result in the continuous accumulation of QDs and may amplify the effect of prophage activation at very low concentrations. Moreover, in aquatic ecosystems, both metal-based NPs and their released metal ions can be taken up by aquatic organisms and be further bioaccumulated and biomagnified.2 Technology exists to increase the stability and to make QDs more biocompatible through coating with protective polymers or several biomolecules.60 However, over long periods of time under local acid or alkaline conditions, QDs can lose their coating and become destabilized.61,62 Notably, recent findings suggested that the level of ROS induced by nanomaterials will increase several-fold after their exposure to light,6,63 and thus their prophage induction effects could be amplified. Nanomaterials that were previously considered biocompatible or environmentally friendly may also be able to induce prophages due to the presence of light-activated redox species. After this initial study, more efforts should be taken to analyze the potential environmental risks of nanomaterials toward the activation of prophages and toward the disruption of ecosystem functions by killing beneficial bacteria in ecosystems.
Conclusions
In this work, spherical CdTe QDs with a size of circa 2 nm were synthesized by the aqueous synthesis method using the coating ligands MPA and GSH. The prophage induction activity of CdTe QDs was investigated for the first time. The mechanism of the prophage induction activity of CdTe QDs was found to be the strand breakage of bacterial DNA. Oxidative stress induced by the QDs was found to be crucial for the prophage induction activity, while the contribution of Cd2+ release was relative lower. Antioxidants provided a significant stronger protective effect on the prophage induction activity than the metal-ion chelator. This paper will deepen the understanding of effects of QDs on microbial environments and provide new concerns for the potential environmental risks of quantum dots.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (21473125 and 21773178), the Natural Science Foundation of Hubei Province (2018CFA090 and 2014CFA003), the National Infectious Diseases Project (2018ZX10301405), the Guangxi Science and Technology Project (GuiKeAD17195081) and the Bagui Scholar Program of Guangxi Province.References
- V. Biju, Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging drug delivery and therapy, Chem. Soc. Rev., 2014, 43, 744–764 RSC.
- N. C. Mueller and B. Nowack, Exposure modeling of engineered nanoparticles in the environment, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of nanomaterials in the environment, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef PubMed.
- J. Lee, S. Mahendra and P. J. Alvarez, Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations, ACS Nano, 2010, 4, 3580–3590 CrossRef PubMed.
- C. Peng, W. Zhang, H. Gao, Y. Li, X. Tong, K. Li, X. Zhu, Y. Wang and Y. Chen, Behavior and Potential Impacts of Metal-Based Engineered Nanoparticles in Aquatic Environments, Nanomaterials, 2017, 7, 21 CrossRef PubMed.
- C. M. Courtney, S. M. Goodman, J. A. McDaniel, N. E. Madinger, A. Chatterjee and P. Nagpal, Photoexcited quantum dots for killing multidrug-resistant bacteria, Nat. Mater., 2016, 15, 529–534 CrossRef PubMed.
- X. Han, L. Lai, F. Tian, F. L. Jiang, Q. Xiao, Y. Li, Q. Yu, D. Li, J. Wang and Q. Zhang, Toxicity of CdTe quantum dots on yeast Saccharomyces cerevisiae, Small, 2012, 8, 2680–2689 CrossRef PubMed.
- M. Bottrill and M. Green, Some aspects of quantum dot toxicity, Chem. Commun., 2011, 47, 7039–7050 RSC.
- J. Kloepfer, R. Mielke, M. Wong, K. Nealson, G. Stucky and J. Nadeau, Quantum dots as strain-and metabolism-specific microbiological labels, Appl. Environ. Microbiol., 2003, 69, 4205–4213 CrossRef PubMed.
- L. Lai, S.-J. Li, J. Feng, P. Mei, Z.-H. Ren, Y.-L. Chang and Y. Liu, Effects of Surface Charges on the Bactericide Activity of CdTe/ZnS Quantum Dots: A Cell Membrane Disruption Perspective, Langmuir, 2017, 33, 2378–2386 CrossRef PubMed.
- C. Howard-Varona, K. R. Hargreaves, S. T. Abedon and M. B. Sullivan, Lysogeny in nature: mechanisms, impact and ecology of temperate phages, ISME J., 2017, 11, 1511–1520 CrossRef PubMed.
- C. Canchaya, C. Proux, G. Fournous, A. Bruttin and H. Brüssow, Prophage genomics, Microbiol. Mol. Biol. Rev., 2003, 67, 238–276 CrossRef PubMed.
- J. Choi, S. M. Kotay and R. Goel, Various physico-chemical stress factors cause prophage induction in Nitrosospira multiformis 25196-an ammonia oxidizing bacteria, Water Res., 2010, 44, 4550–4558 CrossRef PubMed.
- S. Bloch, B. Nejman-Faleńczyk, J. M. Łoś, S. Barańska, K. Łepek, A. Felczykowska, M. Łoś, G. Węgrzyn and A. Węgrzyn, enes from the exo–xis region of λ and Shiga toxin-converting bacteriophages influence lysogenization and prophage induction, Arch. Microbiol., 2013, 195, 693–703 CrossRef PubMed.
- C. Janion, Inducible SOS response system of DNA repair and mutagenesis in Escherichia coli, Int. J. Biol. Sci., 2008, 4, 338 CrossRef PubMed.
- K. Norregaard, M. Andersson, K. Sneppen, P. E. Nielsen, S. Brown and L. B. Oddershede, Effect of supercoiling on the λ switch, Bacteriophage, 2014, 4, e27517 CrossRef PubMed.
- J. H. Paul, Prophages in marine bacteria: dangerous molecular time bombs or the key to survival in the seas?, ISME J., 2008, 2, 579 CrossRef PubMed.
- L. McDaniel and J. Paul, Effect of nutrient addition and environmental factors on prophage induction in natural populations of marine Synechococcus species, Appl. Environ. Microbiol., 2005, 71, 842–850 CrossRef PubMed.
- T. Maskow, B. Kiesel, T. Schubert, Z. Yong, H. Harms and J. Yao, Calorimetric real time monitoring of lambda prophage induction, J. Virol. Methods, 2010, 168, 126–132 CrossRef PubMed.
- T. C. Johnstone, S. M. Alexander, W. Lin and S. J. Lippard, Effects of monofunctional platinum agents on bacterial growth: A retrospective study, J. Am. Chem. Soc., 2013, 136, 116–118 CrossRef PubMed.
- R. Brayner, R. Ferrari-Iliou, N. Brivois, S. Djediat, M. F. Benedetti and F. Fiévet, Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium, Nano Lett., 2006, 6, 866–870 CrossRef PubMed.
- M. Aye, C. Di Giorgio, I. Berque-Bestel, A. Aime, B. P. Pichon, Y. Jammes, P. Barthelemy and M. De Meo, Genotoxic and mutagenic effects of lipid-coated CdSe/ZnS quantum dots, Mutat. Res., 2013, 750, 129–138 Search PubMed.
- Y. Guo, C. Cheng, J. Wang, Z. Wang, X. Jin, K. Li, P. Kang and J. Gao, Detection of reactive oxygen species (ROS) generated by TiO2 (R), TiO2 (R/A) and TiO2 (A) under ultrasonic and solar light irradiation and application in degradation of organic dyes, J. Hazard. Mater., 2011, 192, 786–793 CrossRef PubMed.
- E. V. Skorb, D. V. Andreeva, A. P. Raiski, N. A. Belyasova, H. Mohwald and D. V. Sviridov, Titanium dioxide-assisted photocatalytic induction of prophages to lytic cycle, Photochem. Photobiol. Sci., 2011, 10, 1974–1978 Search PubMed.
- M. Filipic, T. Fatur and M. Vudrag, Titanium dioxide-assisted photocatalytic induction of prophages to lytic cycle, Hum. Exp. Toxicol., 2006, 25, 67–77 Search PubMed.
- A. Wang and D. E. Crowley, Global gene expression responses to cadmium toxicity in Escherichia coli, J. Bacteriol., 2005, 187, 3259–3266 CrossRef PubMed.
- X. Xiang, C. Wu, B.-R. Zhang, T. Gao, J. Zhao, L. Ma, F.-L. Jiang and Y. Liu, The relationship between the length of surface ligand and effects of CdTe quantum dots on the physiological functions of isolated mitochondria, Chemosphere, 2017, 184, 1108–1116 CrossRef PubMed.
- W. W. Yu, L. Qu, W. Guo and X. Peng, Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals, Chem. Mater., 2003, 15, 2854–2860 CrossRef.
- J. Xu, B. Kiesel, R. Kallies, F. e. Jiang, Y. Liu and T. Maskow, A fast and reliable method for monitoring of prophage-activating chemicals, Microb. Biotechnol., 2018 Search PubMed.
- D. Lillehaug, An improved plaque assay for poor plaque-producing temperate lactococcal bacteriophages, J. Appl. Microbiol., 1997, 83, 85–90 Search PubMed.
- X. Li, L. Xu, W. Zhou, Q. Zhao and Y. Wang, Chronic exposure to microcystin-LR affected mitochondrial DNA maintenance and caused pathological changes of lung tissue in mice, Environ. Pollut., 2016, 210, 48–56 CrossRef PubMed.
- C. Liu, K. Gong, X. Mao and W. Li, Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma, Int. J. Cancer, 2011, 129, 1519–1531 CrossRef PubMed.
- J. Xu, F. L. Jiang, Y. Liu, B. Kiesel and T. Maskow, An enhanced bioindicator for calorimetric monitoring of prophage-activating chemicals in the trace concentration range, Eng. Life Sci., 2018 DOI:10.1002/elsc201800026.
- S. P. Brown, L. L. Chat, M. D. Paepe and F. Taddei, Ecology of microbial invasions: amplification allows virus carriers to invade more rapidly when rare, Curr. Biol., 2006, 16, 2018–2052 Search PubMed.
- J.-C. Jin, X.-J. Wu, J. Xu, B.-B. Wang, F.-L. Jiang and Y. Liu, Ultrasmall silver nanoclusters: Highly efficient antibacterial activity and their mechanisms, Biomater. Sci., 2017, 5, 247–257 RSC.
- T. Maskow, R. Kemp, F. Buchholz, T. Schubert, B. Kiesel and H. Harms, What heat is telling us about microbial conversions in nature and technology: from chip to megacalorimetry, Microb. Biotechnol., 2010, 3, 269–284 CrossRef PubMed.
- R. Moldovan, E. Chapman-McQuiston and X. L. Wu, On kinetics of phage adsorption, Biophys. J., 2007, 93, 303–315 CrossRef PubMed.
- T. Maskow, F. M. Morais, L. F. M. Rosa, Y. G. Qian and F. Harnisch, Insufficient oxygen diffusion leads to distortions of microbial growth parameters assessed by isothermal microcalorimetry, RSC Adv., 2014, 4, 32730–32737 RSC.
- D. Mo, L. Hu, G. Zeng, G. Chen, J. Wan, Z. Yu, Z. Huang, K. He, C. Zhang and M. Cheng, Cadmium-containing quantum dots: properties, applications, and toxicity, Appl. Microbiol. Biotechnol., 2017, 101, 2713–2733 CrossRef PubMed.
- A. Pramanik, D. Laha, D. Bhattacharya, P. Pramanik and P. Karmakar, A novel study of antibacterial activity of copper iodide nanoparticle mediated by DNA and membrane damage, Colloids Surf., B, 2012, 96, 50–55 CrossRef PubMed.
- F. Caldefie-Chézet, S. Walrand, C. Moinard, A. Tridon, J. Chassagne, M.-P. Vasson, F. Caldefie-Chézet, S. Walrand and C. Moinard, et al., Is the neutrophil reactive oxygen species production measured by luminol and lucigenin chemiluminescence intra or extracellular? Comparison with DCFH-DA flow cytometry and cytochrome c reduction, Clin. Chim. Acta, 2002, 319, 9–17 CrossRef.
- Z. Lu, C. M. Li, H. Bao, Y. Qiao, Y. Toh and X. Yang, Mechanism of antimicrobial activity of CdTe quantum dots, Langmuir, 2008, 24, 5445–5452 CrossRef PubMed.
- A. D. de Azevedo Neto, J. T. Prisco, J. Enéas-Filho, C. E. B. de Abreu and E. Gomes-Filho, Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes, Environ. Exp. Bot., 2006, 56, 87–94 CrossRef.
- J. Nordberg and E. S. J. Arnér, The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones, Free Radical Biol. Med., 2001, 31, 1287–1312 CrossRef PubMed.
- T. Finkel and N. J. Holbrook, Oxidants, oxidative stress and the biology of ageing, Nature, 2000, 408, 239–247 CrossRef PubMed.
- D. Steinberg, Low density lipoprotein oxidation and its pathobiological significance, J. Biol. Chem., 1997, 272, 20963–20966 CrossRef PubMed.
- F. M. El-Demerdash, M. I. Yousef and F. M. Radwan, Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs, Food Chem. Toxicol., 2009, 47, 249–254 CrossRef PubMed.
- D. Del Rio, A. J. Stewart and N. Pellegrini, A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress, Nutr., Metab. Cardiovasc. Dis., 2005, 15, 316–328 CrossRef PubMed.
- X.-Y. Fan, X.-Y. Chen, Y.-J. Liu, H.-M. Zhong, F.-L. Jiang and Y. Liu, Oxidative stress-mediated intrinsic apoptosis in human promyelocytic leukemia HL-60 cells induced by organic arsenicals, Sci. Rep., 2016, 6, 29865 CrossRef PubMed.
- W. W. Yu, L. Qu, W. Guo and X. Peng, Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals, Chem. Mater., 2003, 15, 2854–2860 CrossRef.
- G. Karniadakis, A. Beskok and N. Aluru, Microflows and nanoflows, Springer, New York, 2005 Search PubMed.
- K. A. Soni, A. K. Balasubramanian, A. Beskok and S. D. Pillai, Zeta potential of selected bacteria in drinking water when dead, starved, or exposed to minimal and rich culture media, Curr. Microbiol., 2008, 56, 93–97 CrossRef PubMed.
- E. Oh, R. Liu, A. Nel, K. B. Gemill, M. Bilal, Y. Cohen and I. L. Medintz, Meta-analysis of cellular toxicity for cadmium-containing quantum dots, Nat. Nanotechnol., 2016, 11, 479–486 CrossRef PubMed.
- R. Werlin, J. H. Priester, R. E. Mielke, S. Krämer, S. Jackson, P. K. Stoimenov, G. D. Stucky, G. N. Cherr, E. Orias and P. A. Holden, Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain, Nat. Nanotechnol., 2011, 6, 65–71 CrossRef PubMed.
- M. S. Cooke, M. D. Evans, M. Dizdaroglu and J. Lunec, Oxidative DNA damage: mechanisms, mutation, and disease, FASEB J., 2003, 17, 1195–1214 CrossRef PubMed.
- O. Choi, K. K. Deng, N.-J. Kim, L. Ross, R. Y. Surampalli and Z. Hu, The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth, Water Res., 2008, 42, 3066–3074 CrossRef PubMed.
- M. Auffan, J. Rose, J.-Y. Bottero, G. V. Lowry, J.-P. Jolivet and M. R. Wiesner, Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective, Nat. Nanotechnol., 2009, 4, 634–641 CrossRef PubMed.
- S. K. Manna, S. Sarkar, J. Barr, K. Wise, E. V. Barrera, O. Jejelowo, A. C. Rice-Ficht and G. T. Ramesh, Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-κB in human keratinocytes, Nano Lett., 2005, 5, 1676–1684 CrossRef PubMed.
- P. C. Naha, M. Davoren, F. M. Lyng and H. J. Byrne, Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells, Toxicol. Appl. Pharmacol., 2010, 246, 91–99 CrossRef PubMed.
- D. M. Aruguete, J. S. Guest, W. W. Yu, N. G. Love and M. F. Hochella, Environ. Chem., 2010, 7, 28 CrossRef.
- S. Mahendra, H. Zhu, V. L. Colvin and P. J. Alvarez, Quantum dot weathering results in microbial toxicity, Environ. Sci. Technol., 2008, 42, 9424–9430 CrossRef PubMed.
- D. R. Cooper, N. M. Dimitrijevic and J. L. Nadeau, Hormonal regulation of glucose production and utilization. Studies with radioactive glucose, Nanoscale, 2010, 2, 114–121 RSC.
- W. Zhang, Y. Li, J. Niu and Y. Chen, Photogeneration of reactive oxygen species on uncoated silver, gold, nickel, and silicon nanoparticles and their antibacterial effects, Langmuir, 2013, 29, 4647–4651 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8en00142a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |