Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emission and fate modelling framework for engineered nanoparticles in urban aquatic systems at high spatial and temporal resolution
Prado
Domercq
 *a,
Antonia
Praetorius
*a,
Antonia
Praetorius
 *bc and
Alistair B. A.
Boxall
*bc and
Alistair B. A.
Boxall
 a
a
aEnvironmental Department, University of York, Heslington, York, YO10 5DD, UK. E-mail: prado.domercq@york.ac.uk
bDepartment of Environmental Geosciences and Environmental Science Research Network, University of Vienna, Althanstr. 14, 1090 Vienna, Austria. E-mail: antonia.praetorius@univie.ac.at
cResearch Platform Nano-Norms-Nature, University of Vienna, 1090 Vienna, Austria
First published on 3rd January 2018
Abstract
Trends in global urbanization and technology development have raised concerns about the associated increase in emissions to the environment, including novel contaminants such as engineered nanoparticles (ENPs). The assessment of these emissions in urban systems requires modelling approaches that integrate the complexity of urban environments as well as the high spatial and temporal variability of contaminant emissions. ENPs are emitted to urban surface waters through a variety of point and diffuse sources, with these emissions being driven by weather, usage patterns and population density. While the potential environmental and health impacts of ENPs are still not fully understood, understanding the spatial and temporal distribution of ENPs at the local scale will help to inform risk assessment. In this paper, we propose a novel modelling approach for estimating the exposure of ENPs in surface waters of urban systems. An integrative modelling framework combining an emission and a fate model for ENPs with high spatial and temporal resolution is presented and strategies for data gathering and the handling of knowledge gaps are discussed. Our framework is capable of identifying local emission hot spots and predicting exposure across a city, while generating information on the final speciation of the emitted ENPs (nano form, aggregates and other transformation products) within the studied environmental compartments over time.
Environmental significanceExisting modelling approaches for assessing environmental exposure to engineered nanoparticles at the local level do not have the necessary temporal and spatial resolution. Assessing exposure at high resolution is important in complex environments, such as cities, where large spatial and temporal differences in exposure are expected due to variability in population density, land cover characteristics and human activity, as well as in wastewater and surface water flows. Here, we present a modelling framework that can be used to model exposure across urban aquatic systems over time. The availability of this modelling framework will allow us to much better characterise nanoparticle exposure through identification of the most relevant pollution sources and will therefore be invaluable for assessing the environmental risks of nanoparticles in urban settings. |
Introduction
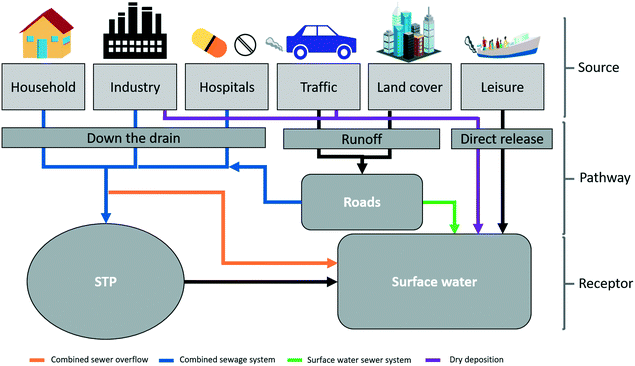
Cities are centres of human activity and consequently represent hot spots of pollutant emissions. With increasing urbanization and the steady growth of the urban population,1 waste and pollution issues arising from cities are becoming more important.2 For example, fresh water supply and wastewater management have become compromised in fast-growing, low-income urban areas where the existing infrastructures and the receiving waters cannot cope with the rise in fresh water demand and the generation of larger volumes of wastewater.3 At the same time, in developed countries, new technological developments and changes in consumer preferences (i.e. development and use of novel materials) together with demographic changes (e.g. ageing and consequent consumption of larger amounts of pharmaceuticals) have raised concerns over the impacts of the so-called emerging pollutants (EPs), which will be emitted to city environments in increasing amounts in the future.4EPs are novel pollutants that are not conventionally monitored and which have the potential to enter the environment and cause adverse effects on ecosystems and human health.4 Examples of EPs include pharmaceutical residues, personal care products (PCPs), engineered nanoparticles (ENPs) and microplastics (MPs). The appearance of PCPs in surface waters5,6 and of pharmaceutical residues in wastewater, surface, ground and drinking water in and around cities has already been proven,7 and signs of adverse ecological effects have been observed.8 However, for certain EPs such as ENPs and MPs, appropriate analytical methods for their detection in environmental matrices are still under development, making large-scale monitoring campaigns currently difficult or impossible.4 Over the last twenty years ENPs have become more and more prevalent on the market due to their demonstrated advantages over conventional chemicals. These new materials bring a wide range of benefits, including improved energy efficiency, material use reduction, and better performance in many existing and new applications.9 They are already incorporated into a wide variety of products spanning various urban sectors (industry, households, buildings, traffic, leisure activities) and are likely emitted to urban surface waters through diffuse (runoff and leaching) and point sources (wastewater treatment plants, surface water overflows).10 Further expansion of the nanotechnology sector is forecasted by the latest report published on Global Nanotechnology Outlook 2022 (ref. 11) meaning that concentrations in the environment are set to continue increasing. Therefore, approaches to quantify local ENP emissions and exposures will be needed. Currently, acceptable exposure levels of ENPs in surface waters in terms of risk are under debate, while the overall picture of ENP emissions and fate in urban environments remains unclear.10,12
The inherent characteristics of urban environments differ significantly from natural environments and they represent unique reactors that will influence ENP transformation and transport. An interplay of various natural and anthropogenic factors will affect ENP emissions and fate in cities (Fig. 1). The complexity of sewage networks (with separated and combined systems), specific characteristics of wastewater treatment plants, geographical and meteorological conditions together with the variety of land cover types, predominant activities and specific regulations present in cities, need to be assessed. Temporal variations in the emissions of ENPs and exposure at the local scale are also expected due to dependencies on weather events and usage patterns; the same apply to the spatial variation of emissions and exposure. This was shown in the study carried out by Gottschalk et al., 2011,13 where they found high temporal and spatial variability in the local predicted environmental concentrations (PECs) determined for several ENPs and pointed out the location-time dependency for their risk assessment. More recent studies by Dumont et al., 2014 (ref. 14) and Dale et al., 2015 (ref. 15) also emphasize the importance of understanding the spatial and temporal variations of ENP concentrations in surface waters.
 | ||
| Fig. 1 Representation of the main factors influencing the emission of pollutants, including ENPs, in urban environments. | ||
Clearly, the estimation of ENP exposure in urban surface waters is a complex challenge and, in order to understand potential environmental impacts of ENPs in urban settings, exposure concentrations need to be assessed at high temporal and spatial resolution. To address this challenge, mathematical models emerge as powerful tools that can be used to estimate ENP emissions and environmental concentrations and provide an indication of what the environment is exposed to when experimental and monitoring data is missing.12,16,17 Furthermore, models enable a deeper understanding of the relative importance of different emission sources and pathways and therefore can serve as tools to support decision making and prioritize regulations. For example, when studying emission mitigation measures, models can help develop more targeted monitoring campaigns once potential hot spots are identified. Models can also help inform the development of analytical methodologies for ENPs by helping to define which material types most need to be monitored and the required performance of the methods in terms of detection limits for particle number and particle size.
Several different modelling approaches for deriving PECs of ENPs have been developed over the last 10 years.16,18,19 Since Boxall et al., 2007,20 provided the first approach and theoretical basis to quantitatively assess ENPs concentrations in air, soil and water using a series of algorithms, ENP emission and fate models have been in constant evolution. From approaches based on hypothetical ENP production and use volumes (when no empirical studies had yet been performed),20 to material flow analysis (MFA)21 or particle flow analysis (PFA),22 to the most recent incorporation of probabilistic elements using Monte Carlo simulations.23 The existing models cover different spatial scales (global, regional and local) as well as different environmental compartments (sediments, soils, atmosphere, surface water, sewage treatment plant effluents and sewage treatment sludge), however more recent models can integrate various compartments24 and spatial scales.25 They also vary in complexity depending on the number of uses targeted for a single ENP (from one single use to the consideration of the full product life cycle) and the incorporation of nanoparticle-specific environmental fate processes. Models can be classified into two main categories: top-down models, in which the environmental compartment is typically treated as a black box and no specific information about the ENP fate processes is included (mainly MFA models), and the more mechanistic bottom up models (environmental fate models), which include relevant information on the ENP transport and fate processes such as advective transport, (hetero-) aggregation, dissolution, surface transformations, sedimentation and resuspension.14,17,27–29,52 In a recent review by Nowack, 2017,16 a summary of the available models of both types (material flow and environmental fate models) is presented together with the identified knowledge gaps. A series of recommendations to enable exposure models to be used from a regulatory perspective, are also presented. Within the modelling framework that we present here, we aim to integrate some of these recommendations: creating a better link between emission estimation and fate models and the inclusion of temporal and spatial resolution.
In the context of urban environments, no nanoparticle-specific models have been developed yet and several key factors are essential for performing a comprehensive urban exposure assessment. These factors need to be targeted within an integrated urban ENP model framework. Firstly, on the local scale of a city, high spatial and temporal resolution data on ENP emissions is required to account for all the source variabilities present in an urban environment (traffic, industry, leisure, etc.), as well as for the local temporal variations in emissions (weather influence, activity dynamics, etc.) and to ultimately provide highly resolved local exposure patterns. Most of the currently available ENP emission models base their ENP emission estimations on global or regional production and usage rates (generally obtained from market reports and other peer-reviewed studies),25,30 and the dynamics of the emissions are only rarely considered.26,31 In terms of spatial resolution, the values of estimated emissions volumes are usually provided as average figures per environmental compartment (natural: water, soil, air; or technical: sewage sludge, landfill, etc.) rather than spatially resolved estimates. Secondly, the use of a bottom up mechanistic approach for the ENP emissions is also missing as an input for existing ENP environmental fate models. As previously stated, those models include nano-specific transport and fate process descriptors, however, they usually rely on averaged production and emission volumes and transfer coefficients derived from MFA models.25,30 In the urban context, we believe that very specific usage patterns of the ENP-containing products can be obtained and that these will influence the ENP release mechanisms and final emissions. More detailed process descriptors are needed to replace the averaged transfer coefficients values used so far in MFA models. This can be achieved by developing release pathway-specific emission equations parametrized with product-specific release rates. Finally, since ENP behaviour is characterized by kinetically dominated transformation and transport processes (e.g. aggregation, sedimentation, dissolution, surface transformations) and is affected by the physical and chemical properties of the surrounding environment (surface water flow, pH, ionic content, UV exposure, etc.), a high spatial and temporal resolution of ENP fate processes is needed to account for the spatial and temporal variations in these parameters occurring within the urban environment.
Here, we propose a novel and comprehensive urban exposure modelling framework for assessing exposure to ENPs in urban aquatic environments based on a source-pathway-receptor structure (Fig. 2). The framework uses a bottom up approach where PEC values can be derived from a detailed study of the emission, transport and fate mechanisms of ENPs contained in products used in cities. By considering all different ENP emission sources, all the identified release pathways, the temporal dynamics of those emissions, as well as the urban and environmental parameters influencing ENP fate processes, the framework presented here is able to estimate exposure at high spatial and temporal resolution. At this local scale the main interest is to be able to identify hot spots of emission and exposure across the city, including the final speciation of the emitted ENPs (nano form, aggregates and other transformation products) within the studied environmental compartments over time.
Model framework for ENPs in urban environments
The proposed modelling framework for estimating exposure to ENPs in urban environments brings together two separated but interconnected models:• an emission estimation model, that estimates the emission rates of specific ENP-containing products used in cities at high spatial and temporal resolution; and
• a surface water fate model that provides the final surface water concentrations (also in a spatially and temporally resolved way) for the parent ENPs and any transformation products (e.g. aggregates), taking into account the transport and fate processes that the ENPs undergo once released to the water compartment.
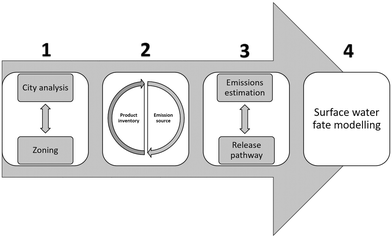
Both models employ a bottom up approach where emission equations and fate processes are based on the specific usage patterns, release pathways, transformation and transport behaviour of the ENP-containing products used within the city. The framework includes the use of monitored chemical and physical characteristics of the targeted surface water(s) in the city of interest to parametrize the fate processes. High spatial and temporal resolution is achieved using spatially resolved city information, geographic information system (GIS) tools and local weather patterns. The proposed framework builds on four mains steps (summarized in Fig. 3) to obtain highly resolved predictions of exposure for ENPs in the urban surface water system:
1. City analysis – urban zoning and river reach delimitation: in order to obtain the required high local spatial resolution, we propose a subdivision of the urban area of study into so-called hydrological zones, and a further delimitation of the surface water bodies into river reaches (or other surface water sections, e.g. for lakes). ENP emission rates can then be obtained per hydrological zone and specific ENP exposure concentrations can be obtained for each of the connected river reaches.
2. Nano product inventory (NPI): a product inventory of the currently used products containing ENPs in the studied urban area is developed and a preliminary classification is performed based on their probable sources of emission. A database with relevant information on these products (usage rates, market penetration, etc.) and the properties of the contained ENPs (size distribution, concentration, surface properties/coatings, etc.) should be included in the inventory where available.
3. Emission estimation model (EEM): to obtain the ENP emission rates to each river reach, calculations based on the source of emission, release pathway and release dynamics are performed. For that purpose, the specific ENP release mechanisms from the specific product usage pattern (bottom up approach) and the emission pathway mechanisms are studied and the emission equation designed accordingly.
4. Surface water fate model (SWFM): surface water exposure concentrations for specific ENPs in each river reach are obtained from a simulation of the fate processes that will occur for ENPs in the surface water system.
By employing this approach based on spatial information on usage and environmental parameters specific to a certain city it is possible to generate spatially and temporally explicit exposure estimations for ENPs in the studied urban surface water system. In the next sections, the individual steps are described in more detail.
1. City analysis: urban zoning and river reaches delimitation
ENPs will be emitted to urban environments from point (wastewater generated in households, hospitals and industry) and diffuse sources (traffic emissions or weathering of urban land cover material for example). By knowing the potential emission sources and their localization within the studied city, and performing a spatial analysis of the area, a high spatial resolution understanding of the emissions can be achieved and a map of emissions across the city can be obtained.We propose a subdivision of the studied urban area into so-called hydrological zones, which contain all sections of the city's sewage network and smaller surface water bodies that flow into a previously defined specific river reach. In this way, ENP emissions are estimated per hydrological zone (enabling the identification of hot spots of emission within the city area) and their distribution along the surface water systems can be tracked based on the hydrological zone-river reach connections. The subdivision into hydrological zones and river reaches is done using GIS tools and by analysing two main data sets: the digital elevation map (DEM) of the area (digital elevation data of the area derived from surveys carried out by remote sensing) and the city drainage network maps (sewage networks maps of the city containing the combined, surface and foul drainage networks, as well as combined sewer overflow (CSO) and storm water outlets (SWOs) locations). Information on the location of the sewage treatment plants (STPs) serving the area and their discharge points, as well as the localization of industrial activities will also guide the hydrological zone delimitation.
The DEM of the area provides information on the water flow directions and sub-catchment areas of the city based on the elevation of the terrain analysed. This information guides the delimitation of areas of the city with confluent flows of runoff water and the identification of their discharge points along the river (hydrological zone-river reach connections). Additionally, the city drainage network maps provide information on the connectivity of the different areas of the city to the local STPs and the location of CSO and SWO, so that the path of the wastewater and collected runoff water can be fully tracked. Thereby, the hydrological zone delimitation is guided by the extension of the areas of confluent flows of runoff waters and also by their connectivity to specific wastewater treatment plants. In this sense the wastewater generated by the households of one specific hydrological zone will all be connected to the same wastewater drainage system and directed to the same STP, and the runoff water generated in that same hydrological zone will all discharge into the same river reach. The criteria for the river reach delimitation are based in the mentioned connections as well as the location of water parameters monitoring locations. As an example, the subdivision for the city of York (UK) into hydrological zones is presented in Fig. 4.
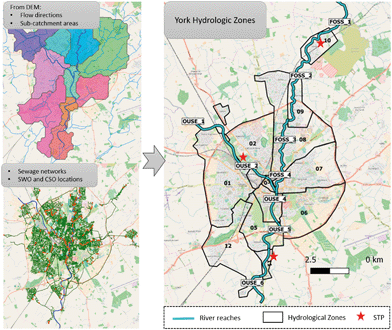 | ||
| Fig. 4 Subdivision of the York area of study into hydrological zones, delimitation of its rivers (Ouse and Foss) into river reaches and localization of the local sewage treatment plants (STPs). | ||
Following this approach, ENP emission estimates can be obtained in a spatially resolved manner. For example, ENPs emitted from point sources can be tracked by knowing the specific location of the STP and CSO outlets (given by the water management local authorities). For the ENP emitted from diffuse sources and through runoff, the emissions' spatial distribution will depend on the terrain elevation (which determines the runoff waters flow directions) as well as on sewer infrastructure (localization and sewer system type and capacity of the area studied).
2. Nano product inventory and classification of emission sources
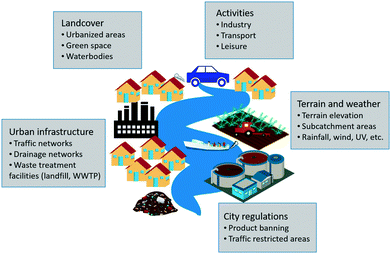
In the urban context, nanotechnology is widely applied. ENPs are integrated into a wide variety of daily use products such as cosmetics, textiles, foods and paints and can be found in different outdoor urban materials such as building façade paints, wood coatings, self-cleaning glass, photocatalytic concrete pavements, and more.10 Furthermore, urban nanotechnology is seen as a potential source of solutions for a sustainable urban development: ranging from providing more resilient and durable construction materials with the potential of decreasing the urban heat-island effect, to contributing to the production of solar power generated by nano-photovoltaic systems, to improving water and air quality through the use of nano-based photocatalytic applications or fuel combustion ENP-catalysts that reduce exhaust emissions.32,33To evaluate the urban ENP exposure, all the potential ENP emission sources must be identified. Therefore, all potential ENP-containing products commercialized and used in the targeted city need to be investigated and classified according to these emission sources. The main categories of ENP emission sources identified for urban environments are briefly presented in Fig. 2 and explained in the following list:
• Household: this category includes all products used indoors that would be released down the drain together with normal wastewater. Product types within the household category include clothing, cosmetics, cleaning products and food additives.
• Industry: ENPs manufactured on site (depending on the industries present in the studied city) will be included in this category as well as other ENPs that might be used in industrial processes (catalysts, fuel additives, cleaning products, etc.).
• Hospitals: ENPs are currently used in medical applications and can consequently be released from hospitals or patients during a hospital stay or in their households.
• Traffic: ENP-containing products that might be released to streets and roads due to traffic (i.e. by exhaust emission and deposition) are included in this category. Examples include ENP-based fuel additives, ENPs generated due to tyre abrasion or ENP-containing products that are used for car maintenance such as car wax or car paint.
• Land cover: this category includes all ENP-containing products used outdoors and that will potentially release ENPs through weathering with rainfall. Examples of these products are paints and other outdoor urban coating products, ENP-containing construction materials (photocatalytic glass, solar panels, cement etc.) as well as ENPs used in novel nano-enabled agricultural technologies such as nano-pesticides and nano-fertilizers that can be used in urban gardens and road verges.
• Leisure: ENP-containing products that might be used during and for leisure activities such as sunscreens or boat paints fall under this category.
Table 1 provides a summary of the ENP-containing products most relevant to urban environments that will fall within the different emission source categories as well as the types of ENPs that can be integrated in the products. This list was developed by combining information gathered from the scientific literature and nano-product inventories such as the Nanotechnology Consumer products inventory (CPI), developed by the Woodrow Wilson International Centre for Scholars and the Project on Emerging nanotechnologies,34 and the most recent Nanodatabase.35 It is worth noting that Table 1 not only contains products already on the market (and identifiable as containing ENPs by the Nanodatabase), but also covers potential future applications of ENPs in urban environments (e.g. within building material or in medical applications, marked in the table by *). Once the emission sources have been identified, additional information is required for the evaluation of their relevance and quantification. Generally, the market penetration of the product (in terms of ratio of ENP-containing product available on the market vs. non nano options) and product type usage (amount of product used per capita in the selected time range) will be the two main factors to consider when estimating the relevance of various emissions in the city investigated. For example, in a southern European, coastal city, where the water leisure activities may be the main drivers of the economy, the market penetration and usage rates of products such as sunscreens will be higher than in cities with no seaside and colder weather. Therefore, for the first case the leisure source will be considered as one of the most relevant sources, while in the second city case it might not even be integrated into the emissions analysis. Other city characteristics, such as local or regional regulations regarding the specific use of certain products (i.e. banning of specific products or traffic restrictions in certain city zones), will influence the relevance of studying certain types of ENP emissions for different cities. Also, the presence of industrial areas or urban agricultural areas (with consequent potential use of nano-pesticides or nano-fertilizers) in the city will influence the ENP emission sources to be considered (industry or land cover sources respectively). Other information, relevant for the emission estimation calculations, such as ENP content of the product, composition and size distribution of the ENPs, will also be gathered. Some potential sources for gathering such information are discussed in the next section.
| Emission source | Product type | ENP |
|---|---|---|
| Household | Cosmetics (makeup and hair treatment) | SiO2, TiO2, ZnO, carbon black, Si, Cu |
| Personal care products (toothpaste, deodorants and creams) | Ag, Au, TiO2, ZnO | |
| Clothing | Ag, Si, ZnO | |
| Food additives | TiO2, Cu, Zn, SiO2 | |
| Sunscreens | TiO2, ZnO | |
| Industry | All product types and ENPs listed above and below | |
| Hospitals | Medical nanoformulations* | — |
| Traffic | Fuel additives | CeO2 |
| Cleaning agents | Ag, TiO2, Au | |
| Maintenance products | SiO2, Ti, Au, Ag | |
| Tyres (abrasion of tyres) | ZnO,36 ZnS, carbon black37 | |
| Land cover | Construction materials* | Carbon nanotubes, SiO2, Ag, TiO2, Al2O3 |
| Paints and surface coatings | TiO2, Ag, SiO2, Cu, ZnO | |
| Nano-pesticides and nano-fertilizers* | Cu/CuO | |
| Environmental remediation | Nano zero-valent iron (nZVI) | |
| Leisure | Sunscreens | TiO2, ZnO |
| Paints and surface coatings for boats | TiO2, Ag, SiO2, Cu | |
3. Emission estimation model
The emissions of ENPs from ENP-containing products can occur during the different stages of their life cycle (production, transport, use and disposal)9 and the extent of these emissions will be source and pathway dependent. A schematic representation of the emission sources and identified emission pathways to urban surface waters is summarised in Fig. 2. The three most relevant release pathways for ENPs towards urban surface waters are i) down the drain, ii) runoff and iii) direct release.Some experimental studies have already demonstrated ENP emissions from everyday products. For example, Benn et al. 2010 (ref. 38) found that Ag ENPs integrated in toothpaste, shampoo, medical cloth and other household products were released to different extents down the drain after a 1 hour washing process in tap water. Also, in studies performed by Bossa et al. 2017 (ref. 39) and Kaegi et al. 2008,40 TiO2 ENPs incorporated in building material (self-cleaning cement) and exterior paints respectively, were found in the leachate collected after simulated runoff events leading to emission estimates of 33.5 mg of Ti/m2 and 16.7 mg of Ti/m2. These released ENPs will be emitted to the aquatic environment (wastewater and/or surface waters) in different contexts (outdoors and indoors) depending not only on the specific usage and disposal pattern of the product, but also, in the case of outdoor use products (i.e. in traffic or land cover), on specific weather conditions (i.e. rainfall events leading to weathering of outdoor ENP-containing materials and transport of ENP deposited on the land cover of the city). At the same time, it has been demonstrated that a considerable portion of ENPs emitted with the wastewater (i.e. Ag and CeO2 ENPs for the studies of Kaegi et al. 2011 (ref. 41) and Limbach et al. 2008 (ref. 42) respectively), is retained in the sludge of wastewater treatment plants. A similar process could occur for ENPs emitted with runoff, where ENPs could be retained to some extent in the impervious cover through physical or chemical interactions (i.e. adsorption to concrete). Therefore, to estimate ENP emissions to surface waters, it is not only important to consider the emissions of the ENPs from the source, but also the pathway that the ENPs follow once released from the product to the surface waters (that will determine the retention rates), and the weather conditions that trigger such emissions (in the case of runoff emissions). To estimate emissions of ENPs to surface water for each of the three identified release pathways we have developed the following generic equations:
• Down the drain (wastewater) emissions:
| EWW = PEmiss·FSTP·(1 − cret) + PEmiss·(1 − FSTP)·Tlag | (1) |
• Runoff emissions:
| ERO = PEmiss·(1 − cret)·Tlag | (2) |
• Direct release emissions:
| ED = PEmiss·Tlag | (3) |
A more detailed explanation of the variables integrating eqn (1)–(3) is provided in the next sections.
| PEmiss = CENP·Uprod·Fpen·Rrelease | (4) |
The value of CENP should be obtained either from the product manufacturer (as done by Tiede et al. 2016 (ref. 43)) or from chemical analysis of a product;44 the value of Fpen will be obtained from sources such as consumer product surveys (such as the surveys performed by Tiede et al. 2016 (ref. 43) or Zhang et al. 2015 (ref. 45)), manufacturers surveys and market reports (e.g. Future Markets 2012 used by Keller et al. 2013 (ref. 25)). The values of Uprod will be estimated either by the use of technical guidance documents (such as European Chemicals Bureau, 2003 (ref. 46) used by Tiede et al. 2016 (ref. 43)) or obtained through consumer surveys;47 or by the use of product-specific equations based on the product usage patterns. Finally, Rrelease will be obtained from experimental studies39 or from manufacturers (i.e. ageing and end of life experiments). Some examples of equations for estimating the Uprod for a selection of product types and their corresponding Rrelease are presented in Table 2.
| Product type | U prod (mass or volume of product/time) | R release |
|---|---|---|
| Cosmetics and personal care products | = U·Pop | = fraction of ENPs released from the specific product during usage. (i.e. as worst case scenario 100% of the contained ENPs would be released so Rrelease = 1) |
| U: usage per capita per day | ||
| Pop: population of the area studied | ||
| Fuel additives | = Fj·Nj | = fraction of ENPs released from the specific product during usage (in this case percentage of fuel additive that escapes from the exhaust) |
| F j: fuel consumed (L) per vehicle type (j) per day, | ||
| N j: average number of vehicles of type j circulating in the area studied per day; | ||
| Maintenance products | = U·SAj·Nj | = fraction of ENPs released from the product |
| U: product usage per day per surface area of material exposed | ||
| SA: surface area covered by the product per vehicle type (j) | ||
| N j average number of vehicles of type j circulating in the area studied per day | ||
| Construction materials | = U·SA/τ | = fraction of ENPs released from the product during it lifetime |
| U: mass of product used per surface area of material exposed | ||
| τ: number of days of exposure of the product, in this case lifetime of the construction material (i.e. 10 years, τ = 3650 days) | ||
| SA: building facades surface area exposed to weathering | ||
| Paints and surface coatings | = U·SA/τ | = fraction of ENPs released from the product during its lifetime |
| U: product usage (in mass or volume) per surface area of material exposed | ||
| SA: building facades surface area exposed to weathering | ||
| τ: number of days of exposure of the product, in this case frequency of facade painting (i.e. once every six months, the paint will be exposed and the ENPs released through 6 months, τ = 182 days) |
In the study performed by Tiede et al. 2016,43 where data available for 126 ENP-containing products commercialised in the UK were analysed, quantitative information of the CENP, Uprod and Fpen for 62 of them was found from similar information sources. For example, for cosmetics containing SiO2 ENPs, the CENP value was found from the manufacturers information (labelling) as 15% of the cosmetic composition. The usage was estimated as 0.8 g of product per capita per day by following the ECHA technical guidance 2003 (TGD 2003).46 And Fpen was estimated through the use of a local product survey where the number of nano-containing products was divided by the total amount of products (nano and non nano) available on the same market (0.5% of the skincare market).
In this modelling approach time resolution is given by a lag time factor called Tlag. We generally describe Tlag as the period of delay between the use phase of the product (with potential ENPs release) and the actual discharge of the ENPs into the surface waters. This factor is release pathway dependent in terms.
For example, in runoff processes Tlag is equivalent to the duration of a dry period. During this period of time, while no rainfall events happen, either the ENPs are not emitted (mainly the ones emitted through weathering of surfaces), or they are emitted (mainly to the atmosphere) and deposited and accumulated in the urban surfaces through dry deposition but not discharged in the surface waters until the actual runoff emission occurs during a rain event. In the case of wastewater emission, Tlag is measured in terms of the average residence time of wastewater in an STP (time that the wastewater spends in the STP without being discharged into the surface water bodies). And for the direct release pathway, Tlag would be zero in the case of leisure activities release (i.e. sunscreens release while bathing), but dependent on the ENPs transport dynamics and weather conditions (wind speed, humidity, etc.) in the case of direct dry deposition. To quantify this explicitly, atmospheric particle transport models would be needed. Kumar et al. 2011,49 present a review on the different available dispersion models that address the dynamics of ENP dispersion in the atmosphere where lag time can be extrapolated from.
Temporal variations can therefore be integrated within the model by means of Tlag, and the determination of its values will usually be weather dependent. Local water management authorities would be therefore a key information source to establish Tlag values.
Alternatively, higher time resolution could be implemented if desired and if permitted by the computational power, depending on data availability. For example, different patterns of usage and disposal of specific products at different times during the day could be included by the application of other time specific factors to the PEmiss.
| Pathway | c ret | Description |
|---|---|---|
| Down the drain | c stp | The STP removal efficiency for the specific ENP and type of STP in place |
| Runoff | c road | Road retention efficiency for the specific ENP |
| Direct release | — | No retention processes happen in this case |
The values for each cret will need to be obtained from experimental studies, if available, and will be ENP specific.
4. Surface water fate model
While the emission estimates serve to quantify ENP loads towards urban surface waters, environmental fate processes will determine the final concentration and distribution of ENPs within the water bodies (Fig. 5). Furthermore, the environmental impact and health risks associated with exposure to ENPs will be strongly affected by the “form” in which the ENPs are present, i.e. whether they are freely dispersed, homo- and/or hetero-aggregated, dissolved or whether they have undergone transformations of their surface or surface coating. ENP concentration and form will be determined by processes such as homo- and heteroaggregation, photochemical transformations, oxidation and reduction, dissolution, precipitation, adsorption/desorption of macromolecules, biotransformation among other biogeochemically driven processes.50 Many environmental factors, biotic and abiotic, play important roles in these transformation and transport processes: ionic strength and composition, pH, water hardness and the presence of dissolved organic matter and suspended particulate matter (SPM) will alter aggregation and transformation processes and are expected to ultimately influence ENPs toxicity by altering their availability for uptake and distribution within organisms, and via interactions with other pollutants.51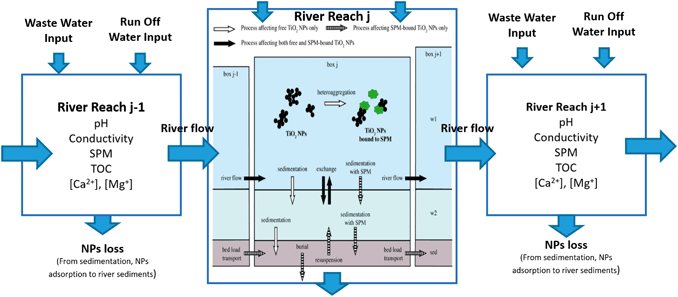 | ||
| Fig. 5 Schematic representation of the ENP transport into, out of and throughout the defined river reaches and fate processes inside the river system. Adapted from Praetorius et al. 2012.52 | ||
To account for the importance of environmental fate processes on the final exposure estimates of ENPs a comprehensive model framework for ENPs in surface waters needs to integrate a mechanistic surface water fate model where relevant transport and fate processes such as advective transport, sedimentation, resuspension, hetero-aggregation and dissolution of ENPs are simulated at high spatial and temporal resolution. A modular multimedia-box model including nanoparticle-specific process descriptions for water and sediments, such as the one developed by Praetorius et al. 2012,52 can be directly linked to the emission estimation model. This river model can be easily adjusted and parameterized to represent the properties (e.g. dimensions and discharge) of the specific river(s) or lake(s) of a given city. In the model, spatial resolution is provided by the subdivision of the model into individual boxes, each of which is divided into three compartments (stagnant and flowing water compartments and sediment compartment). This model approach takes into account ENP-specific properties (composition, size, density, attachment efficiencies for aggregation etc.) to parametrise their fate processes in an aquatic medium. Variations in aquatic properties (discharge, water depth, pH, water composition etc.) can be included by subdividing the river model into individual sections of distinct conditions. For optimal parameterization of the model, we recommend to accompany the model development with a local monitoring campaign of surface waters to provide actual data on the key water parameters (e.g. pH, conductivity, ion concentration and concentrations of dissolved organics and suspended solids) which will affect the fate and distribution of ENPs around the surface water system of the city.
5. Challenges in model parametrization
As seen throughout the modelling framework description, a vast amount of data is required for full parametrization of the modelling approach. Table 4 summarises examples of data that could feed into each of the modelling framework steps, as well as some potential data sources.| Framework steps | Related data | Sources |
|---|---|---|
| 1. City analysis | Surface water distribution, flow directions, catchment areas (digital elevation maps, surface water maps) | Governmental geographical or environmental agencies (e.g. USGS, EEA, EPA, etc.). National or regional mapping agencies (i.e. ordnance survey) |
| Urban wastewater and surface water distribution and connections with surface water bodies (city drainage network maps, STP and CSO locations) | City council urban planning department or local water management companies (e.g. Yorkshire Water Ltd.) | |
| ENP emission source locations and distribution in the studied area (location of industrial areas, STPs, traffic networks, leisure areas, etc.) | City council urban planning department, open source city council resources (e.g. YorkOpenData) and/or regional mapping agencies (e.g. ordnance survey) | |
| 2. NPI | List of commercialized ENP-containing products | Online nano-product inventories (i.e. CPI,34 Nanodatabase35), commercial, consumer and industrial surveys55 and market studies (i.e. Global Nanotechnology Market Outlook 2022 (ref. 11)) |
| Manufacturer specifications, technical guidelines,46 consumer55 and market surveys, market reports11 | ||
| ENP-containing products information (CENP, Uprod, Fpen, Rrelease) | When no data is available usage rates can be extrapolated from life cycle assessment studies (e.g. usage of ENP contained in fuel additives can be estimated from traffic data and vehicle performance as specified in Table 2) | |
| ENP characteristics (size distribution, surface/coating properties, etc.) | Manufacturer specifications, patent registry or in situ lab analysis of the product | |
| 3. EEM | Release rates from products or applications | Experimental studies (i.e. release of TiO2 ENPs from paints,40 release of CeO2 ENPs from fuel additives application56) |
| Sewage fraction to go to connected STP (FSTP) | Local water management companies (e.g. Yorkshire Water Ltd.) | |
| Retention coefficient (cret) | Experimental studies (e.g. Kaegi et al. 2011 (ref. 41) and Limbach et al. 2008 (ref. 42)) | |
| Lag time (Tlag) | Local water management companies, local weather stations (e.g. Yorkshire Water Ltd.) and ENP dynamic atmospheric transport models49 | |
| 4. SWFM | Water parameters (pH, flow, ionic strength, etc.) | Water quality monitoring campaigns (environmental agencies or performed independently) |
| ENP characteristics (size distribution, composition, etc.) | Product specifications from manufacturers, or product analysis |
The model parametrization is indeed one of the biggest challenges in exposure modelling.16 This is because experimental data is often scarce or missing, especially in the case of emerging pollutants, such as ENPs, that are not conventionally monitored or regulated. To date, solely some nano-specific provisions in product-centric regulations (e.g. EU cosmetics, food information and biocide regulations) for products containing nanomaterials exists53,54 But, uniformity in ENP regulations in terms of product labelling and notification requirements is still lacking, which makes the estimation of ENP emissions, based on production and usage volumes, hard to perform.
Therefore, new strategies have to be found in order to bridge those data gaps. Some strategies are described within this modelling framework, where we propose the use of a bottom up approach for the determination of usage and release rates at the local level (Table 2). Local data, such as traffic patterns for the estimation of ENP-traffic related emissions, or local weather information for the estimation of local release rates of ENPs imbibed in materials exposed to weathering such as outdoor paints, are more easily accessible and accurate than using average ENP production estimates and steady-state release coefficients for example.
Additionally, it is worth noting that one of the advantages of modelling is its flexibility in terms of scenario analysis. In this sense different tiers of assessment are always possible. For example, one could start with a rather rough estimation, using market data for consumption and averaged environmental conditions and later move to a more refined assessment (as data becomes available), using data collected specifically for the given city through the use of local consumption surveys and local water monitoring data. These different tiers can be assessed at different resolution, both in terms of space and time.
Conclusions
The modelling framework presented here has been designed to serve as a guide for estimating exposure of urban environments to ENPs. Taking into account the complexity of such systems and the level of local resolution targeted, it is worth pointing out that the quality of the PECs estimated will highly depend on the data availability and quality for the city studied. Our framework proposes an integrated methodology to follow but has been designed in a highly flexible way so that it can be adapted to various types of cities and be workable for different levels of data availability. Furthermore, this urban modelling framework can be easily adjusted to other types of emerging pollutants, being particularly suited for other particulate contaminants such as microplastics. The modular nature of the framework makes it very versatile in terms of its inherent flexibility to integrate additional modules or release pathways that have not yet been identified but that could become relevant in the study of other emerging pollutants.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Yorkshire Water Services Limited for its support in this project. This research is part of Cutting-edge Approaches for Pollution Assessment in Cities (CAPACITIE) project, which has received funding from the European Union's Seventh Framework Programme for research, technological development, and demonstration under grant agreement no 608014.References
- P. D. (2014), United Nations, Department of Economic and Social Affairs and H. ST/ESA/SER. A. World Urbanization Prospects: The 2014 Revision, World Urbanization Prospects, The 2014 Revision, 2014.
- A. Pal, Y. He, M. Jekel, M. Reinhard and K. Y. H. Gin, Environ. Int., 2014, 71, 46–62 CrossRef CAS PubMed.
- S. K. Karn and H. Harada, Environ. Manage., 2001, 28, 483–496 CrossRef CAS PubMed.
- V. Geissen, H. Mol, E. Klumpp, G. Umlauf, M. Nadal, M. van der Ploeg, S. E. A. T. M. van de Zee and C. J. Ritsema, Int. Soil Water Conserv. Res., 2015, 3, 57–65 CrossRef.
- J. M. Brausch and G. M. Rand, Chemosphere, 2011, 82, 1518–1532 CrossRef CAS PubMed.
- X. Peng, Y. Yu, C. Tang, J. Tan, Q. Huang and Z. Wang, Sci. Total Environ., 2008, 397, 158–166 CrossRef CAS PubMed.
- T. Heberer, K. Reddersen and A. Mechlinski, in Water Science and Technology, 2002, vol. 46, pp. 81–88 Search PubMed.
- J. O. Tijani, O. O. Fatoba and L. F. Petrik, Water, Air, Soil Pollut., 2013, 224, 1770 CrossRef.
- A. A. Keller, S. McFerran, A. Lazareva and S. Suh, J. Nanopart. Res., 2013, 15, 1692 CrossRef.
- M. Baalousha, Y. Yang, M. E. Vance, B. P. Colman, S. McNeal, J. Xu, J. Blaszczak, M. Steele, E. Bernhardt and M. F. Hochella, Sci. Total Environ., 2016, 557–558, 740–753 CrossRef CAS PubMed.
- Research and Markets, Global Nanotechnology Market Outlook 2022, 2015, (http://www.researchandmarkets.com/reports/3512791/global-nanotechnology-market-outlook-2022).
- L. Duester, M. Burkhardt, A. C. Gutleb, R. Kaegi, A. Macken, B. Meermann and F. von der Kammer, Front. Chem., 2014, 2, 39 Search PubMed.
- F. Gottschalk, C. Ort, R. W. Scholz and B. Nowack, Environ. Pollut., 2011, 159, 3439–3445 CrossRef CAS PubMed.
- E. Dumont, A. C. Johnson, V. D. J. Keller and R. J. Williams, Environ. Pollut., 2015, 196, 341–349 CrossRef CAS PubMed.
- A. L. Dale, G. V. Lowry and E. A. Casman, Environ. Sci. Technol., 2015, 49, 7285–7293 CrossRef CAS PubMed.
- B. Nowack, NanoImpact, 2017, 8, 38–47 CrossRef.
- N. Sani-Kast, M. Scheringer, D. Slomberg, J. Labille, A. Praetorius, P. Ollivier and K. Hungerbühler, Sci. Total Environ., 2015, 535, 150–159 CrossRef CAS PubMed.
- F. Gottschalk, T. Y. Sun and B. Nowack, Environ. Pollut., 2013, 2013, 287–300 CrossRef PubMed.
- A. L. Dale, E. A. Casman, G. V. Lowry, J. R. Lead, E. Viparelli and M. Baalousha, Environ. Sci. Technol., 2015, 49, 2587–2593 CrossRef CAS PubMed.
- A. Boxall, Q. Chaudhry, C. Sinclair, A. Jones, R. Aitken, B. Jefferson and C. Watts, Current and Future Predicted Environmental Exposure To Engineered Nanoparticles, 2007 Search PubMed.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 44447–44453 CrossRef.
- R. Arvidsson, S. Molander and B. A. Sanden, J. Ind. Ecol., 2012, 16, 343–351 CrossRef CAS.
- F. Gottschalk, R. W. Scholz and B. Nowack, Environ. Model. Softw., 2010, 25, 320–332 CrossRef.
- J. A. J. Meesters, J. T. K. Quik, A. A. Koelmans, A. J. Hendriks and D. van de Meent, Environ. Sci.: Nano, 2016, 3, 715–727 RSC.
- A. A. Keller and A. Lazareva, Environ. Sci. Technol. Lett., 2013, 1, 65–70 CrossRef.
- A. Praetorius, R. Arvidsson, S. Molander and M. Scheringer, Environ. Sci.: Processes Impacts, 2013, 15, 161–168 CAS.
- A. L. Dale, E. A. Casman, G. V. Lowry, J. R. Lead, E. Viparelli and M. Baalousha, Environ. Sci. Technol., 2015, 49, 2587–2593 CrossRef CAS PubMed.
- J. J. M. De Klein, J. T. K. Quik, P. S. Bäuerlein and A. A. Koelmans, Environ. Sci.: Nano, 2016, 3, 434–441 RSC.
- A. A. Markus, J. R. Parsons, E. W. M. Roex, P. de Voogt and R. W. P. M. Laane, Water Res., 2016, 91, 214–224 CrossRef CAS PubMed.
- A. A. Keller, S. McFerran, A. Lazareva and S. Suh, J. Nanopart. Res., 2013, 15, 1692–1694 CrossRef.
- T. Y. Sun, G. Conroy, E. Donner, K. Hungerbühler, E. Lombi and B. Nowack, Environ. Sci.: Nano, 2015, 2, 340–351 RSC.
- A. Wiek, D. Guston, S. van der Leeuw, C. Selin and P. Shapira, J. Urban Technol., 2013, 20, 45–62 CrossRef.
- V. A. M. Selvan, R. B. Anand and M. Udayakumar, J. Eng. Appl. Sci., 2009, 4, 1–6 CrossRef.
- M. E. Vance, T. Kuiken, E. P. Vejerano, S. P. McGinnis, M. F. Hochella and D. R. Hull, Beilstein J. Nanotechnol., 2015, 6, 1769–1780 CrossRef CAS PubMed.
- S. Foss Hansen, L. Roverskov Heggelund, P. Revilla Besora, A. Mackevica, A. Boldrin and A. Baun, Environ. Sci.: Nano, 2016, 3, 169–180 RSC.
- Y. Yang, M. Vance, F. Tou, A. Tiwari, M. Liu and M. F. Hochella, Atmos. Environ., 2014, 2, 534–544 Search PubMed.
- P. Kumar, L. Pirjola, M. Ketzel and R. M. Harrison, Atmos. Environ., 2013, 67, 252–277 CrossRef CAS.
- T. Benn, B. Cavanagh, K. Hristovski, J. D. Posner and P. Westerhoff, J. Environ. Qual., 2010, 39, 1875–1882 CrossRef CAS PubMed.
- N. Bossa, P. Chaurand, C. Levard, D. Borschneck, H. Miche, J. Vicente, C. Geantet, O. Aguerre-Chariol, F. M. Michel and J. Rose, Environ. Pollut., 2017, 220, 1160–1170 CrossRef CAS PubMed.
- R. Kaegi, A. Ulrich, B. Sinnet, R. Vonbank, A. Wichser, S. Zuleeg, H. Simmler, S. Brunner, H. Vonmont, M. Burkhardt and M. Boller, Environ. Pollut., 2008, 156, 233–239 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, B. Sinnet, S. Zuleeg, H. Hagendorfer, M. Burkhardt and H. Siegrist, Environ. Sci. Technol., 2011, 45, 3902–3908 CrossRef CAS PubMed.
- L. K. Limbach, R. Bereiter, E. Muller, R. Krebs, R. Galli and W. J. Srtark, Environ. Sci. Technol., 2008, 42, 5828–5833 CrossRef CAS PubMed.
- K. Tiede, S. Foss Hanssen, P. Westerhoff, G. J. Fern, S. M. Hankin, R. J. Aitken, Q. Chaudhry and A. B. A. Boxall, NANO, 2016, 10, 1743–5390 Search PubMed.
- N. S. Tulve, A. B. Stefaniak, M. E. Vance, K. Rogers, S. Mwilu, R. F. LeBouf, D. Schwegler-Berry, R. Willis, T. A. Thomas and L. C. Marr, Int. J. Hyg. Environ. Health, 2015, 218, 345–357 CrossRef CAS PubMed.
- Y. Zhang, Y. R. Leu, R. J. Aitken and M. Riediker, Int. J. Environ. Res. Public Health, 2015, 12, 8717–8743 CrossRef CAS PubMed.
- European Commission - Joint Research Centre - Institute for Health and Consumer Protection, Technical Guidance Document on Risk Assessment, 2003 Search PubMed.
- A. A. Keller, W. Vosti, H. Wang and A. Lazareva, J. Nanopart. Res., 2014, 16, 2489 CrossRef.
- T. Y. Sun, D. M. Mitrano, N. A. Bornhöft, M. Scheringer, K. Hungerbühler and B. Nowack, Environ. Sci. Technol., 2017, 51, 2854–2863 CrossRef CAS PubMed.
- P. Kumar, M. Ketzel, S. Vardoulakis, L. Pirjola and R. Britter, J. Aerosol Sci., 2011, 42, 580–603 CrossRef CAS.
- B. Nowack, J. F. Ranville, S. Diamond, J. A. Gallego-Urrea, C. Metcalfe, J. Rose, N. Horne, A. A. Koelmans and S. J. Klaine, Environ. Toxicol. Chem., 2012, 31, 50–59 CrossRef CAS PubMed.
- R. D. Handy, R. Owen and E. Valsami-Jones, Ecotoxicology, 2008, 17, 315–325 CrossRef CAS PubMed.
- A. Praetorius, M. Scheringer and K. Hungerbühler, Environ. Sci. Technol., 2012, 46, 6705–6713 CrossRef CAS PubMed.
- V. Amenta, K. Aschberger, M. Arena, H. Bouwmeester, F. B. Moniz, P. Brandhoff, S. Gottardo, H. J. Marvin, A. Mech, L. Q. Pesudo and H. Rauscher, Regul. Toxicol. Pharmacol., 2015, 73, 463–476 CrossRef PubMed.
- D. M. Bowman, G. van Calster and S. Friedrichs, Nat. Nanotechnol., 2010, 5, 92 CrossRef CAS PubMed.
- C. Dimitroulopoulou, E. Lucica, A. Johnson, M. R. Ashmore, I. Sakellaris, M. Stranger and E. Goelen, Sci. Total Environ., 2015, 536, 880–889 CrossRef CAS PubMed.
- G. E. Batley, B. Halliburton, J. K. Kirby, C. L. Doolette, D. Navarro, M. J. Mclaughlin and C. Veitch, Environ. Toxicol. Chem., 2013, 32, 1896–1905 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |