Size- and surface charge-controlled layered double hydroxides for efficient algal flocculation†
Tae-Hyun
Kim
 ,
In Taek
Hong
and
Jae-Min
Oh
,
In Taek
Hong
and
Jae-Min
Oh
 *
*
Department of Chemistry & Medical Chemistry, College of Science & Technology, Yonsei University, Wonju, Gangwon-do 26493, Republic of Korea. E-mail: jaemin.oh@yonsei.ac.kr
First published on 28th November 2017
Abstract
We evaluated the effects of particle size and surface charge on the algal flocculation activity of layered double hydroxides (LDHs). LDHs with different lateral sizes of 50, 250, and 2000 nm were prepared by adjusting reaction methods and parameters during co-precipitation of a metal solution under alkaline conditions. LDH particles of 2000 nm readily flocculated ∼100% of Microcystis aeruginosa within 60 min, whereas smaller LDHs did not show significant algal flocculation. We controlled the surface charge of 2000 nm LDHs to be highly positive, almost neutral, or negative using organic moiety coatings of L-serine, succinate, and citrate, respectively. Negatively coated LDHs did not flocculate algae, suggesting the importance of surface charge in the algae–LDH interaction. Taking into account the size and surface charge parameters for algal flocculation, we prepared LDHs with a lateral size of ∼500 nm and a positive surface charge from industrial waste slag, a by-product of alloy manufacturing, from an electric arc furnace. These LDHs showed ∼40% flocculating activity within 60 min, whereas the slag ground into powder showed little flocculating activity after 240 min.
Environmental significanceAlgal bloom is a serious environmental problem in fresh water. Many techniques have been developed to remove algae, and sedimentation upon adsorption is practically applied. Generally utilized adsorbents include clays but their adsorption efficiency is not optimized yet. Based on the hypothesis that the algal–adsorbent interaction is governed by surface interaction, we fine-tuned the particle size and surface charge of synthetic clays to investigate algal flocculation. We found that the particle size (∼2000 nm) and surface charge (positive) are key factors that control algal–clay flocculation. Considering applicability, we suggested a method to prepare synthetic clays with large size and positive charge from industrial waste slag. The obtained clay powder showed a much higher algal flocculation than the conventional clays. |
Introduction
Harmful algal blooms (HABs), which are attributed to global warming and nutrient superabundance in fresh water, are a serious environmental problem in many countries, including the United States, China, and South Korea.1–5 Among the various blue-green algae that can cause a HAB, Microcystis aeruginosa (MA) causes serious damage to aquatic ecosystems and human life because it produces toxins as secondary metabolites.6–8To reduce HABs, various algal removal techniques have been developed, such as flocculation,9–12 ultrasonic aggregation,13,14 froth flotation,15,16 filtration,17,18 and algicide treatment.19,20 Among these, flocculation is the most practical because of its ease of handling and cost-effectiveness.3 In fact, yellow loess, a natural clay, is currently used to treat HABs in South Korea and Japan,21–23 and many efforts have been made to improve its functionality in algal flocculation.12,24 Clay materials can prohibit the motility of blue-green algae through particle–cell interactions that result in flocculation and effective removal.25 Thus, the first step towards optimizing the functionality of algal flocculating clays is sufficient comprehension of the surface interaction between blue-green algae and its flocculants and how that interaction varies with the physicochemical properties of clay. We here describe the relationships between the physicochemical properties of clay and algal flocculation activity. Studies on the interaction between microorganisms and inorganic nanoparticles are not simply an issue in algal flocculation. In the recent decade, many scientists tried to understand the interaction between small organisms and inorganic nanoparticles in order to develop antibacterial agents,26,27 drug delivery systems28 and vaccine adjuvants.29,30 Therefore, the current study on algal–clay interaction would be scientifically important to develop various bio-functionalized materials.
A layered double hydroxide (LDH), also known as anionic clay, is composed of positively charged nanolayers (M(II)1−xM(III)x(OH)2x+; M(II), M(III): divalent and trivalent metal cations, 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) < x <
< x <![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4) and charge-compensating interlayer anions.31,32 General clays, such as kaolinites and smectites, have fixed physicochemical properties because they are usually obtained from nature and purified. On the other hand, the physicochemical properties of LDHs, such as the particle size, surface charge, and composition, can be easily controlled by adjusting the synthetic conditions. Thus, LDHs can be good model materials for studying the relationship between a clay's properties and algal flocculation. In this study, we prepared LDHs with a well-defined lateral size and surface charges using reagent grade chemicals and evaluated the relationships between these physicochemical properties and algal flocculation activity with MA cells. Then we tried to synthesize an LDH algal flocculant from slag, a by-product of steel or alloy manufacturing. Taking into account that industry produces approximately 50 million tons33 of slag annually, and that the annual consumption of algal flocculant is up to hundreds of thousands of tons,34 converting slag into an algal flocculant would result in a high-value industry. Because it has hydraulic properties and contains various metal species (Fe, Mn, Ca, Mg, Al, and Si), slag has been used as a road base material, a concrete admixture, for soil improvement, or as calcium silicate fertilizer, as-produced or ground to a powder.35–37 However, these applications are quite simple and add little value considering the useful metal components. Several improved applications that use slag for heavy metal adsorption,38 phosphate removal,39 and marine forest restoration40 are in progress. However, the undefined chemical structure and inhomogeneous composition of slag limit their versatility.33 Recently, it was reported that blast furnace slag can be a source for LDH synthesis by extracting metal components via acid treatment.41 Inspired by that work, we analyzed the physicochemical properties of electric arc furnace slag and used it to synthesize LDHs. We then studied the MA flocculation activity of the LDHs in terms of the lateral size and surface charge, the effects of which we had established using LDHs with well-controlled parameters.
0.4) and charge-compensating interlayer anions.31,32 General clays, such as kaolinites and smectites, have fixed physicochemical properties because they are usually obtained from nature and purified. On the other hand, the physicochemical properties of LDHs, such as the particle size, surface charge, and composition, can be easily controlled by adjusting the synthetic conditions. Thus, LDHs can be good model materials for studying the relationship between a clay's properties and algal flocculation. In this study, we prepared LDHs with a well-defined lateral size and surface charges using reagent grade chemicals and evaluated the relationships between these physicochemical properties and algal flocculation activity with MA cells. Then we tried to synthesize an LDH algal flocculant from slag, a by-product of steel or alloy manufacturing. Taking into account that industry produces approximately 50 million tons33 of slag annually, and that the annual consumption of algal flocculant is up to hundreds of thousands of tons,34 converting slag into an algal flocculant would result in a high-value industry. Because it has hydraulic properties and contains various metal species (Fe, Mn, Ca, Mg, Al, and Si), slag has been used as a road base material, a concrete admixture, for soil improvement, or as calcium silicate fertilizer, as-produced or ground to a powder.35–37 However, these applications are quite simple and add little value considering the useful metal components. Several improved applications that use slag for heavy metal adsorption,38 phosphate removal,39 and marine forest restoration40 are in progress. However, the undefined chemical structure and inhomogeneous composition of slag limit their versatility.33 Recently, it was reported that blast furnace slag can be a source for LDH synthesis by extracting metal components via acid treatment.41 Inspired by that work, we analyzed the physicochemical properties of electric arc furnace slag and used it to synthesize LDHs. We then studied the MA flocculation activity of the LDHs in terms of the lateral size and surface charge, the effects of which we had established using LDHs with well-controlled parameters.
Experimental
Materials
Reagent grade Mg(NO3)2·6H2O, Al(NO3)3·9H2O, NaHCO3, CO(NH2)2, trisodium citrate (Na3C6H5O7), disodium succinate (C4H4Na2O4·6H2O), L-serine (C3H7NO3), NaNO3, K2HPO4, H3BO3, MnCl2·4H2O, ZnSO4·7H2O, Na2MoO4·2H2O, CuSO4·5H2O, Co(NO3)2·6H2O, glutaraldehyde solution Grade I, kaolinite, and sepiolite were purchased from Sigma-Aldrich Co. LLC (St. Louis., USA). NaOH pellets, HCl, Na2CO3, and 1,1,1,3,3,3-hexamethyldisilazane (HMDS) were obtained from Daejung Chemicals & Metals Co., Ltd (Siheung, Korea). MgSO4·7H2O, CaCl2·2H2O, ammonium ferric(III) citrate green ((NH4)5[Fe(C6H4O7)2]), and Na2EDTA were purchased from Junsei Chemical Co., Ltd (Tokyo, Japan). Yellow loess was purchased from Green Bio Co., Ltd (Incheon, Korea). All the chemicals were used without further purification.Synthesis of size- and surface charge-controlled LDHs from reagents
LDHs with lateral sizes of ∼50 nm (LDH-1) and ∼250 nm (LDH-2) were prepared by conventional co-precipitation with and without hydrothermal treatment, respectively. A mixed metal solution (0.6 mol L−1 Mg2+ and 0.2 mol L−1 Al3+) was titrated with an alkaline solution (1.8 mol L−1 NaOH and 1.3 mol L−1 NaHCO3) until the pH reached ∼9.5. This slurry was aged at room temperature for 24 h (LDH-1) or hydrothermally treated at 150 °C for 24 h (LDH-2). An LDH with a lateral size of ∼2000 nm (LDH-3) was prepared by the urea hydrolysis method. An aqueous solution containing Mg2+/Al3+/urea (molar ratio = 2/1/3.3) was reacted at 90 °C for 24 h to homogenously hydrolyze urea and precipitate the LDH. The obtained precipitates were transferred to an autoclave and aged at 150 °C for 24 h. The final product was centrifuged, washed several times with deionized water, and then lyophilized.To control the surface charge of LDH-3 to be negative, moderate, or highly positive (preservation of the pristine surface charge), we applied coatings of trisodium citrate, disodium succinate, and L-serine, respectively. First, 0.5 g of pristine LDH powder was dispersed into 200 mL of a solution containing one of the three organic coating agents (0.025 mol L−1) and reacted for 12 h at room temperature. The products were collected by centrifugation, washed several times with deionized water, and then lyophilized.
Preparation of LDHs from slag
Electric arc furnace slag obtained from FeMn alloy manufacturing was provided by Dongil Industries Co., Ltd (Pohang, Korea). The slag was ground into a fine powder using an agate mortar. The powdered slag (5 g) was dispersed into 100 mL of 3 mol L−1 HCl and vigorously stirred for 4 h at 100 °C. Then the suspension was filtered to obtain a supernatant with various metal cations. The supernatant was titrated with an alkaline solution (1.5 mol L−1 of NaOH) until the pH was ∼11.5 and then aged for 24 h at 65 °C. The final product was collected by centrifugation, washed several times with deionized water, and then dried.Microorganisms and culture
Cyanobacteria MA (M. aeruginosa AG20763) was purchased from Korean Collection for Type Cultures (KCTC), Korea. MA cells were cultured in cell culture flasks with 250 mL of BG11 medium prepared by mixing the following: 100 mL NaNO3 (15 mg mL−1), 10 mL K2HPO4 (4 mg mL−1), MgSO4·7H2O (7.5 mg mL−1), CaCl2·2H2O (3.6 mg mL−1), citric acid (0.6 mg mL−1), ammonium ferric(III) citrate green (0.6 mg mL−1), Na2EDTA (0.1 mg mL−1), Na2CO3 (2 mg mL−1), and 1 mL of a trace metal solution (H3BO3 (2.86 mg mL−1), MnCl2·4H2O (1.81 mg mL−1), ZnSO4·7 H2O (0.22 mg mL−1), 0.39 g of Na2MoO4·2 H2O (0.39 mg mL−1), CuSO4·5 H2O (0.08 mg mL−1), and Co(NO3)2·6H2O (0.08 mg mL−1)). The pH of the prepared BG11 medium was adjusted to ∼7.5 with 1 mol L−1 HCl or NaOH. All solutions were prepared with sterilized deionized water. The cell culture flasks were incubated at 28 °C at a light intensity of 140 μmol m−2 s−1 (photon flux density) using fluorescent lamps with an automated light/dark cycle of 12 h/12 h.Characterization
The powder X-ray diffraction (XRD) patterns were obtained using a Bruker AXS D2 Phaser (LYNXEYE™ detector; Bruker AXS GmbH, Karlsruhe, Germany) with a 1 mm air-scattering slit, a 0.1 mm equatorial slit, and time step increments of 0.02° and 0.5 s per step. The particle size and morphology of the prepared samples were determined by scanning electron microscopy (SEM) using a Quanta 250 FEG (FEI Company, Hillsboro, OR, USA). For the SEM measurement, the samples were diluted with deionized water to a concentration of ∼0.4 mg mL−1. Then a drop of a suspension was placed on a pre-cleaned Si wafer and dried in a vacuum. The surface of the prepared samples was sputtered with Pt/Pd plasma for 50 s, and the images were collected using a 30 kV accelerated electron beam. For the SEM images of algae, MA cells were centrifuged and fixed with 2% glutaraldehyde solution at 4 °C for 24 h and then washed three times with phosphate buffered saline for 10 min. The samples were sequentially dehydrated in solutions of 50%, 60%, 70%, 90%, 95%, and 100% ethanol (three times) for 10 min each. The dehydrated samples were dried with HMDS twice for 20 min each. The zeta potential and hydrodynamic radius of the LDHs were verified by electrophoretic light scattering and dynamic light scattering using an ELS-Z1000 (Otsuka Electronics, Osaka, Japan). For the measurement, the samples were dispersed (∼0.1 mg mL−1) in deionized water, and the pH values of the prepared colloid suspensions were adjusted to ∼7.5 with 0.01 mol L−1 HCl or NaOH. The zeta potential value and hydrodynamic radius were calculated using the Smoluchowski equation and Marquardt analysis, respectively, which were carried out using the program provided by Otsuka electronics.Evaluation of flocculation activity with the chlorophyll-a determination method
To determine the flocculation activity, we dispersed the LDH powders (250 mg) into a separate 500 mL MA suspension. The suspension was stirred for 4 min at 600 rpm and for 2 min at 100 rpm. Then 50 mL of the LDH/MA suspension supernatant was collected at designated times (0, 10, 30, 60, 120, and 240 min). The supernatant was filtered with a 25 mm GF/C glass fiber filter and treated with 10 mL of acetone (90%). The prepared samples were stored at 4 °C in the dark for 24 h to extract chlorophyll-a. The amount of chlorophyll-a was measured with a UV-vis spectrometer (UV-1800, Shimadzu, Kyoto, Japan) and calculated using the Mitchell & Kiefer equation (eqn (1))| Amount of Chlorophyll-a = 11.85E664 − 1.54E647 − 0.08E630 | (1) |
The flocculation activity was calculated using the following equation:
| Flocculation activity (%) = 1 − (1 − A/B) × 100 | (2) |
(A: the amount of chlorophyll-a in the supernatant at a specific time; B: the amount of chlorophyll-a in the supernatant at 0 min).
Results and discussion
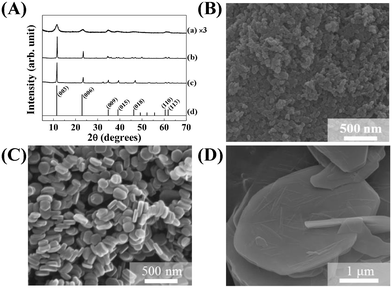
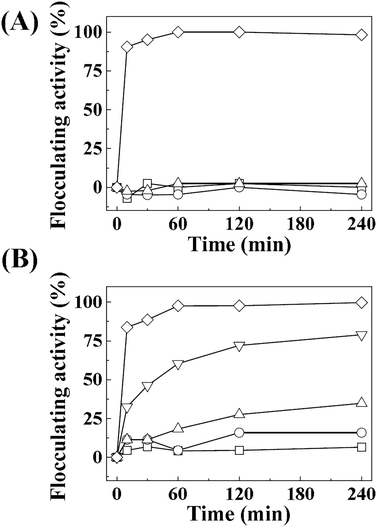
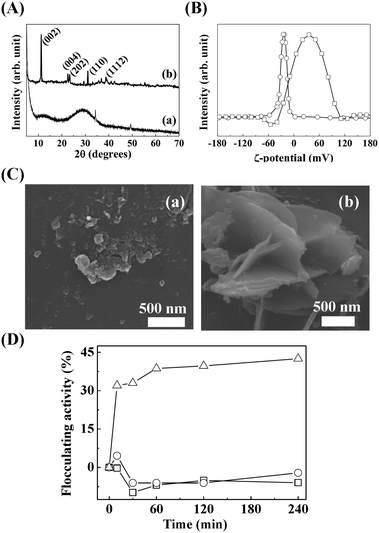
The crystal structure, size, and morphology of the three LDHs were verified by powder XRD and SEM, as shown in Fig. 1. All samples showed typical diffraction patterns corresponding to a hydrotalcite (JCPDS No. 14-0191) structure (Fig. 1(A)), with differences in peak intensity and full width at half maximum depending on the particle size. Larger LDHs showed sharper and more intense diffraction peaks than smaller ones, suggesting a size-crystallinity correlation in LDHs.42 All LDHs exhibited the typical morphology of a layered structure. The average particle sizes calculated from 100 randomly selected particles were 50 ± 2, 270 ± 3, and 2200 ± 41 nm for LDH-1, LDH-2, and LDH-3, respectively. The hydrodynamic radii in the aqueous suspensions were determined to be 90, 250, and 1100 nm for LDH-1, LDH-2, and LDH-3, respectively, suggesting a high correlation between the primary particle size and the hydrodynamic radius. We attribute the smaller hydrodynamic radius (compared to the primary particle size) observed in the SEM image of LDH-3 to its high aspect ratio (lateral dimension/thickness). The average zeta potential values for all LDHs at neutral pH were highly positive: 27.9 ± 0.7, 34.9 ± 0.4, and 32.6 ± 0.7 mV for LDH-1, LDH-2, and LDH-3, respectively (Fig. S1†). The characterization results indicated that the three LDHs differ only in particle size; their crystal phase and surface charge were the same.The size-dependent MA flocculating activity of LDH is displayed in Fig. 2(A). For this experiment, the concentrations of LDHs and cells were fixed to 500 ppm and 1.18 × 107 cells per mL, respectively. The LDH particles of tens or hundreds of nanometers (LDH-1: 50 nm, ○; LDH-2: 270 nm, △) did not show significant flocculating activity compared with the control group (□), even at a long incubation time of up to 240 min. However, LDH-3 (◇), with a lateral size of 2200 nm, flocculated ∼90% of MA within 10 min and almost 100% of MA at 60 min.
Therefore, we evaluated the effect of LDH concentration on algal flocculation using LDH-3 (Fig. 2(B)). The flocculating activity was highly dependent on LDH concentration; 50, 100, 200, and 500 ppm of LDH-3 exhibited 6.5, 15.9, 34.8, 79.0, and 99.7% flocculation, respectively, at 120 min. To evaluate the effect of cell concentration on flocculation, we varied the initial cell numbers while fixing the LDH-3 concentration at 500 ppm. As shown in Table 1, the flocculation ratio decreased as the initial cell number increased, showing 89.0, 50.6, and 25.0% for initial MA cell numbers of 5.90 × 109, 1.19 × 1010, and 2.56 × 1010, respectively. It is worth noting that the number of flocculated MA per 1 mg of LDH was not seriously affected by the initial cell number; the calculated values were 2.17 × 107, 2.42 × 107, and 2.56 × 107 cells per mg LDH-3 for initial MA cell numbers of 5.90 × 109, 1.19 × 1010, and 2.56 × 1010, respectively. Rough calculations indicate that there were 1.1 × 109 particles in 1 mg of LDH-3; thus, approximately 46 LDH-3 particles (calculated from 1.1 × 109 particles per 2.4 × 107 cells) were required to flocculate one cell regardless of the LDH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA ratio.
MA ratio.
| Initial cell number in 500 mL | 5.90 × 109 | 1.19 × 1010 | 2.56 × 1010 |
| Flocculated cell | 5.25 × 109 | 6.05 × 109 | 6.40 × 109 |
| Flocculation ratio (%) | 89.0 | 50.6 | 25.0 |
| Flocculated cells per 1 mg of LDH | 2.1 × 107 | 2.42 × 107 | 2.56 × 107 |
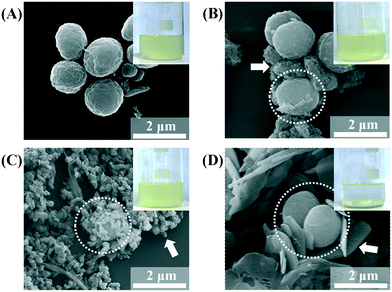
The SEM images of the MA/LDH flocculates showed that the LDH particles covered the MA cells. The MA cells were found to be spherical and 1700 nm in diameter (Fig. 3A). After treatment with LDHs, the MA cells (white dotted circles in Fig. 3B–D) assembled with the LDH particles (white arrows in Fig. 3B–D). We attribute the high algal flocculation activity of LDH-3 to two factors. First, LDHs inherently possess a positive surface charge that facilitates effective adsorption onto the negatively charged algal surface.43 Second, because the zeta potentials of the three LDH samples were similar to one another (Fig. S1†), their different flocculating activities are surely attributable to size. LDH particles tend to agglomerate;44 small LDH particles agglomerated with one another rather than interacting with MA, whereas agglomerates of LDH-3 resulted in a “house-of-cards” structure, whose inter-particle cavity fitted an MA cell (Fig. 3). It could therefore be concluded that LDH-3, which has a lateral dimension comparable to that of MA, could incorporate MA cells through surface–surface interactions more effectively than LDH-1 and LDH-2 (Fig. 3).
Because we found that the LDH particle size was a determining factor for MA flocculation and that the surface charge could also be important, we systematically evaluated the surface charge effect. We controlled the surface charge of LDH-3 to be highly positive, moderately positive, or negative by coating it with L-serine, succinate, and citrate, respectively. All coating agents have a carboxylate moiety that attach to the positive LDH surface. L-Serine, a zwitterionic amino acid with an amine and a carboxylic acid, was thought to preserve the original surface charge of LDH. Succinate and citrate, with two and three carboxylates, respectively, shifted the surface charge negatively. As expected, the zeta potential of citrate-, succinate-, and serine-coated LDHs was well controlled at −35.3 mV, 15.7 mV, and 26.6 mV, respectively. Note that the zeta potential of pristine LDH was 32.6 mV (Fig. 4). There was neither significant alteration of particle size nor serious aggregation of LDH-3 particles upon surface coating, as shown in the SEM images (Fig. 4).
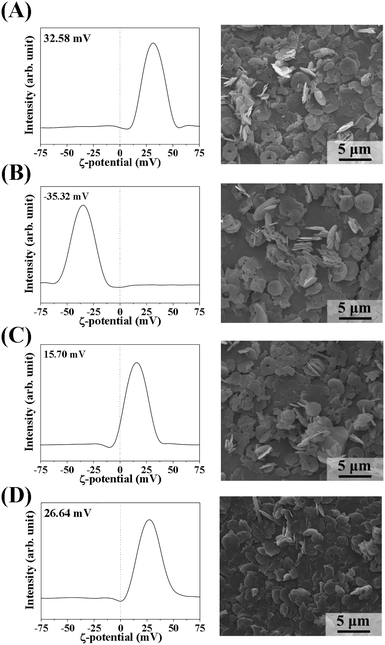 | ||
| Fig. 4 Zeta potential distributions at pH 7.0 (left) and scanning electron microscopy images (right) of (A) LDH-3, (B) LDH-3-cit, (C) LDH-3-suc, and (D) LDH-3-ser. | ||
The algal flocculating activity of surface charge-controlled LDH-3 is displayed in Fig. 5. The results indicate that the flocculation depends on the surface charge. Negatively charged LDH particles did not show effective flocculation, whereas the positive LDH-3-suc and LDH-3-ser exhibited almost the same algal flocculating activity as the uncoated LDH-3.
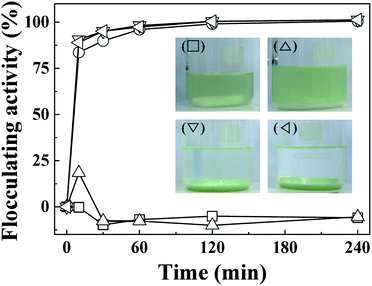
The important role of electrostatic interaction in algal flocculation has been demonstrated in various studies. Metal cations such as trivalent aluminium are widely used to flocculate algae.45,46 Adding positive polymers such as chitosan to negatively charged clays or minerals dramatically enhances the algal removal efficiency.12,24,47 MA cells are known to have abundant anionic moieties (such as carboxylate and phosphoryl) on their surface. Thus, the electrostatic interaction between positive LDH and negative MA forms MA–LDH flocculates. To double check the role of surface charge in algal flocculation, we compared the MA flocculation activity of LDH-3 with that of several representative clay minerals (kaolinite, yellow loess, and sepiolite). The respective zeta potentials of kaolinite, yellow loess, and sepiolite are −34.7 mV, −38.2 mV, and −19.9 mV. In spite of their large hydrodynamic radii (kaolinite: ∼4000 nm, yellow loess: ∼3000 nm, and sepiolite: 4100 nm as dispersed), the algal flocculation activities of these clays were not significant even after 240 min, whereas LDH-3 showed ∼100% efficacy at that time (Fig. 6). Therefore, we conclude that both a large size and a positive surface charge are required for effective algal flocculation.
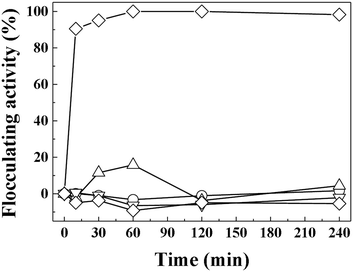 | ||
| Fig. 6 Flocculating activity of LDH-3 (◇), kaolinite (△), yellow loess (▽), sepiolite (○), and negative control (□) on MA algae (1.5 × 107 cells per mL). | ||
Based on our finding that the particle size and surface charge were critical factors for algal flocculation using clay particles, we tried to find a way to recycle industrial waste slag for the high-value added application of algal removal. Slags are by-products generated during iron or steel manufacturing. Because slag is mass produced and causes environmental problems, several recycling methods have been developed, including its use as a contaminant scavenger48,49 and fertilizer and algal flocculation.12,50 However, due to slag's amorphous phase, inhomogeneous distribution of elements, and possible leakage of heavy metal species, its direct application has several drawbacks. Therefore, several groups have tried to synthesize well-defined structures from slag using a series of processes.41,51
After we found that large LDH particles with a positive surface charge were effective in algal removal, we tried to prepare such particles from slag, namely SL, for use as algal flocculants. We determined that the slag in this study consisted mainly of SiO2, MnO2, CaO, MgO, and Al2O3 (Table 2). All metal species except Si can be used in LDH; thus, we extracted metal species from the slag under acidic conditions. The extract was determined to contain Mn, Ca, Mg, and Al. To optimize the pH conditions to precipitate as many metal species as possible into the LDH framework, we investigated the base–pH titration curve for the extract solution (Fig. S2†). There were three plateaus at around pH 4, 8, and 11, which we attributed to the buffering effect during the hydrolysis of Al3+, Mg2+, and Ca2+, respectively. The extract was titrated until pH ∼11.5, which allowed all metal species in the extract (Mn2+/Mn3+, Ca2+, Mg2+ and Al3+) to be effectively incorporated into the LDH framework.
| Materials element | Pristine slag | Leaching solution | SL |
|---|---|---|---|
| Si | 2.97 | N.D. | N.D. |
| Mn | 0.78 | 0.49 | 1.14 |
| Ca | 0.67 | 0.78 | 1.58 |
| Mg | 0.64 | 0.31 | 0.75 |
| Al | 1 | 1 | 1 |
As shown in Fig. 7(A), the XRD pattern of pristine slag showed amorphous peaks from SiO2. On the other hand, SL exhibited sharp (hkl) peaks at 11.24°, 22.65°, 23.51°, and 31.06° that correspond to (002), (004), (202), and (110), respectively, of hydrocalumite (JCPDS No. 31-0245), which is a type of LDH with hepta-coordinated calcium hydroxide.52 The chemical formula of SL, evaluated by ICP-OES and EDS, was CaII1.58MgII0.75AlIII1.00MnIV1.14(OH)8.94Cl3.28·mH2O.
The hydrodynamic radius (data not shown) and zeta potential (Fig. 7B) of the pristine slag and SL were ∼1211 nm at −23.7 mV and ∼470 nm at 33.5 mV, respectively. Although the size was not sufficiently large for effective algal flocculation, the highly positive surface charge of SL is advantageous in algal flocculation. In addition, we expected the hard metal Mn4+ in the SL framework to have a strong affinity to hard bases, such as the carboxyl and phosphoryl on the MA surface. The SEM results show that the prepared SL had a significantly different morphology than the pristine slag (Fig. 7C). The SL showed a 2-dimensional layered morphology with a lateral size of ∼500 nm (Fig. 7), which matched well with the hydrodynamic radius. As shown in Fig. 7D, SL (△) showed moderate flocculation activity of up to ∼45% with 500 ppm treatment, whereas the pristine slag (○) showed no significant flocculation. Our LDH and SL flocculating activity results reveal that a highly positive surface charge and a hydrodynamic radius similar to that of algal cells could enhance flocculation for algal removal.
Conclusions
We have successfully evaluated the effects of the particle size and the surface charge of layered double hydroxides on Microcystis aeruginosa. Various sizes of LDHs were prepared by conventional co-precipitation followed by hydrothermal treatments. From X-ray diffraction and SEM analyses, the prepared LDHs showed a typical hydrotalcite-like structure and defined lateral sizes. Among LDHs of various lateral sizes, 2000 nm sized LDH particles showed ∼100% MA flocculation activity within 60 min. We also controlled the surface charge of 2000 nm LDHs utilizing organic moiety coatings of L-serine, succinate, and citrate, respectively. Negatively charged LDHs showed ∼0% flocculation activity, while positively charged samples showed high flocculation activity, suggesting that the surface charge is one of the important factors for algal flocculation. Also, we prepared LDHs with a lateral size of ∼500 nm and positive surface charges from industrial waste slag. Through MA flocculation experiments, these LDHs showed ∼40% flocculating activity within 60 min. Considering that the lateral size and surface charge are important factors for flocculating algal cells with LDH, LDH from slag could be a candidate for effective algal flocculation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT (NRF-2017R1A2B4006352), and Dongil industries Co., Ltd. This work also supported (in part) by the Yonsei University Research Fund of 2017.References
- D. W. Schindler, Science, 1974, 184, 897–899 CAS.
- P. Hoagland, D. M. Anderson, Y. Kaoru and A. W. White, Estuaries, 2002, 25, 819–837 CrossRef.
- M. R. Sengco and D. M. Anderson, J. Eukaryotic Microbiol., 2004, 51, 169–172 CrossRef CAS.
- D. M. Anderson, Ocean Coast. Manag., 2009, 52, 342–347 CrossRef PubMed.
- K. Zhang, T. F. Lin, T. Zhang, C. Li and N. Gao, J. Environ. Sci., 2013, 25, 1539–1548 CrossRef CAS.
- S. E. Beaulieu, M. R. Sengco and D. M. Anderson, Harmful Algae, 2005, 4, 123–138 CrossRef.
- C. Ross, L. Santiago-Vazquez and V. Paul, Aquat. Toxicol., 2006, 78, 66–73 CrossRef CAS PubMed.
- X. Zhang, H.-Y. Hu, Y.-J. Men, J. Yang and K. Christoffersen, Water Res., 2009, 43, 2953–2960 CrossRef CAS PubMed.
- R. Divakaran and V. N. Sivasankara Pillai, J. Appl. Phycol., 2002, 14, 419–422 CrossRef CAS.
- Y. Tang, H. Zhang, X. Liu, D. Cai, H. Feng, C. Miao, X. Wang, Z. Wu and Z. Yu, Water Res., 2011, 45, 2855–2862 CrossRef CAS PubMed.
- M. Louzao, P. Abal, D. Fernández, M. Vieytes, J. Legido, C. Gómez, J. Pais and L. Botana, Toxins, 2015, 7, 3977 CrossRef CAS PubMed.
- Y. Liu, X. Cao, Z. Yu, X. Song and L. Qiu, J. Appl. Phycol., 2016, 28, 1623–1633 CrossRef CAS.
- X. Wu, E. M. Joyce and T. J. Mason, Water Res., 2012, 46, 2851–2858 CrossRef CAS PubMed.
- G. Yu, C. Zhao, B. Liu, Q. Li and H. Gao, J. Environ. Biol., 2013, 34, 331–335 Search PubMed.
- G. V. Levin, J. R. Clendenning, A. Gibor and F. D. Bogar, Appl. Microbiol., 1962, 10, 169–175 CAS.
- W. Phoochinda and D. A. White, Environ. Technol., 2003, 24, 87–96 CrossRef CAS PubMed.
- Y. Zhang, J. Tian, J. Nan, S. Gao, H. Liang, M. Wang and G. Li, J. Hazard. Mater., 2011, 186, 1415–1424 CrossRef CAS PubMed.
- M. Cerff, M. Morweiser, R. Dillschneider, A. Michel, K. Menzel and C. Posten, Bioresour. Technol., 2012, 118, 289–295 CrossRef CAS PubMed.
- X. Zheng, B. Zhang, J. Zhang, L. Huang, J. Lin, X. Li, Y. Zhou, H. Wang, X. Yang, J. Su, Y. Tian and T. Zheng, Appl. Microbiol. Biotechnol., 2013, 97, 9207–9215 CrossRef CAS PubMed.
- G. Cai, X. Yang, Q. Lai, X. Yu, H. Zhang, Y. Li, Z. Chen, X. Lei, W. Zheng, H. Xu and T. Zheng, Sci. Rep., 2016, 6, 20081 CrossRef CAS PubMed.
- T. Maruyama, R. Yamada, K. Usui, H. Suzuki and T. Yoshida, Nippon Suisan Gakkaishi, 1987, 53, 1811–1819 CrossRef CAS.
- M. Y. Han and W. Kim, Microchem. J., 2001, 68, 157–161 CrossRef CAS.
- X.-X. Sun and J.-K. Choi, Hydrobiologia, 2004, 519, 153–165 CrossRef.
- X. X. Sun, K. N. Han, J. K. Choi and E. K. Kim, Mar. Pollut. Bull., 2004, 48, 937–945 CrossRef CAS PubMed.
- G. Pan, J. Chen and D. M. Anderson, Harmful Algae, 2011, 10, 381–387 CrossRef PubMed.
- I. Y. Kim, S. Park, H. Kim, S. Park, R. S. Ruoff and S.-J. Hwang, Adv. Funct. Mater., 2014, 24, 2257–2257 CrossRef.
- C. Hu, X. Hu, J. Guo and J. Qu, Environ. Sci. Technol., 2006, 40, 5508–5513 CrossRef CAS PubMed.
- J.-M. Oh, S.-J. Choi, G.-E. Lee, J.-E. Kim and J.-H. Choy, Chem. – Asian J., 2009, 4, 67–73 CrossRef CAS PubMed.
- E. Ruiz-Hitzky, M. Darder, P. Aranda, M. Á. M. del Burgo and G. del Real, Adv. Mater., 2009, 21, 4167–4171 CrossRef CAS.
- S. Yan, W. Gu, B. Zhang, B. Rolfe and Z. P. Xu, Dalton Trans., 2017 10.1039/C7DT03725B.
- A. Vaccari, Catal. Today, 1998, 41, 53–71 CrossRef CAS.
- V. Rives, Layered Double Hydroxides: Present and Future, Nova Science Publishers, 2001 Search PubMed.
- C. Navarro, M. Díaz and M. A. Villa-García, Environ. Sci. Technol., 2010, 44, 5383–5388 CrossRef CAS PubMed.
- J. Raloff, http://www.phschool.com/science/science_news/articles/toxic_tides.html.
- W. Cha, J. Kim and H. Choi, Water Res., 2006, 40, 1034–1042 CrossRef CAS PubMed.
- B. Das, S. Prakash, P. S. R. Reddy and V. N. Misra, Resour., Conserv. Recycl., 2007, 50, 40–57 CrossRef.
- P. E. Tsakiridis, G. D. Papadimitriou, S. Tsivilis and C. Koroneos, J. Hazard. Mater., 2008, 152, 805–811 CrossRef CAS PubMed.
- S. V. Dimitrova and D. R. Mehanjiev, Water Res., 2000, 34, 1957–1961 CrossRef CAS.
- E. Oguz, J. Hazard. Mater., 2004, 114, 131–137 CrossRef CAS PubMed.
- T. Kato, C. Kosugi, E. Kiso and K. Torii, Nippon Steel & Sumitomo Metal Tech Rep, 2015, vol. 109, pp. 79–84 Search PubMed.
- Y. Kuwahara, T. Ohmichi, T. Kamegawa, K. Mori and H. Yamashita, J. Mater. Chem., 2010, 20, 5052 RSC.
- J.-M. Oh, S.-H. Hwang and J.-H. Choy, Solid State Ionics, 2002, 151, 285–291 CrossRef CAS.
- K. J. Ives, J. Biochem. Microbiol. Technol. Eng., 1959, 1, 37–47 CrossRef.
- K. Okamoto, N. Iyi and T. Sasaki, Appl. Clay Sci., 2007, 37, 23–31 CrossRef CAS.
- D. Vandamme, I. Foubert, I. Fraeye, B. Meesschaert and K. Muylaert, Bioresour. Technol., 2012, 105, 114–119 CrossRef CAS PubMed.
- N. B. Wyatt, L. M. Gloe, P. V. Brady, J. C. Hewson, A. M. Grillet, M. G. Hankins and P. I. Pohl, Biotechnol. Bioeng., 2012, 109, 493–501 CrossRef CAS PubMed.
- R. S. P. Couto, A. W. S. Guarino, C. W. C. Branco, E. F. A. Palermo and E. G. Azero, Int. J. Environ. Res., 2013, 7, 435–442 CAS.
- P. S. Song, B. Y. Min, W. K. Choi, C. H. Jung and W. Z. Oh, Sep. Purif. Technol., 2008, 60, 136–141 CrossRef CAS.
- V. Z. Serjun, A. Mladenovič, B. Mirtič, A. Meden, J. Ščančar and R. Milačič, Waste Manage., 2015, 43, 376–385 CrossRef CAS PubMed.
- X. Wang and Q.-S. Cai, Pedosphere, 2006, 16, 519–524 CrossRef CAS.
- Y. Kuwahara and H. Yamashita, ISIJ Int., 2015, 55, 1531–1537 CrossRef CAS.
- R. Segni, L. Vieille, F. Leroux and C. Taviot-Guého, J. Phys. Chem. Solids, 2006, 67, 1037–1042 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7en00809k |
| This journal is © The Royal Society of Chemistry 2018 |