Opportunities to advance sustainable design of nano-enabled agriculture identified through a literature review
Jiaoyang
Yin
,
Yan
Wang
 and
Leanne M.
Gilbertson
and
Leanne M.
Gilbertson
 *
*
Department of Civil and Environmental Engineering, University of Pittsburgh, Pittsburgh, PA 15261, USA. E-mail: leanne.gilbertson@pitt.edu; Tel: +412 624 1683
First published on 1st December 2017
Abstract
The application of nanotechnology in agriculture and food systems is a new and rapidly evolving area of research with the potential to positively impact an industry that is experiencing increased demand under increasingly stressed resources. Given the intimate relationship between agriculture, the environment, and human health, a proactive approach to design is critical – one that is informed by considering the trade-offs between potential benefits realized by nano-enabling and potential adverse impacts imposed by their use. This tutorial review includes an overview of current and proposed nano-enabled applications that are unique to agriculture and food systems to identify, (i) the function provided and proposed benefits realized through nano-enabling, (ii) the efficiency of (nano)material use, and (iii) the proposed mechanism through which the ‘nano’ component of the design operates. It is through this review that three primary suggestions emerge, offering guidance for ongoing studies to inform design for enhanced agriculture sustainability: the need for (i) comprehensive data reporting, including material flows (input, emissions, and retention in the environment or product) of the engineered nanomaterials or active ingredient used, (ii) experimental design that includes non-nano controls, and (iii) identification and discussion of mechanisms underlying how the ‘nano’ aspect of the design enables the observed outcome. In addition to overarching guidance for continued research to inform design for enhanced agriculture sustainability, suggestions unique to each reviewed product class are also provided.
Environmental significanceModern agriculture and food system practices suffer from significant inefficiencies that contribute to substantial adverse environmental consequences. Given the projected increase in global demand on an already stressed system, there is a tangible opportunity for engineered nanomaterials and nanotechnologies to have a positive impact in the agriculture sector. As with any new technology, there are trade-offs to consider to ensure development of sustainable solutions. Herein, the current literature on nano-enabled applications in agriculture and food systems is reviewed to identify critical opportunities to guide ongoing research and the sustainable design of nano-enabled agriculture. |
Introduction
The agriculture and food systems sector is and will continue to experience increased pressure resulting from global food demand, strain on natural resources, and the evolution of seasonal climate patterns.1,2 Increased demand for food is inevitable given the current and predicted trend in population growth (9.7 billion by 2050, a 32% increase from 2015, according to the U.N. medium-variant projection),3 including an increase of those with the means and desire for resource intensive foods. An increasingly stressed food supply chain is directly linked to the depletion of natural resources, including energy, water, and non-renewable feedstocks (e.g., fertile soils, sources of primary crop nutrients such as phosphorous), given the current inefficient farming practices.4,5 Unpredictability and increased frequency of extreme climate events (e.g., severe drought, flood conditions) are caused by disruption in seasonal regularity of temperatures and precipitation, which have significant impact on agriculture yield.6–8 In addition, shifts in political, geopolitical, consumer preferences, and consumer behavior all influence the food supply chain. Given the current trajectory, there is significant concern over the security of our future global food supply chain and thus, a need to design and implement lasting sustainable solutions.The utilization of engineered nanomaterials (ENMs) and harnessing of nano-scale phenomena has been suggested as a viable path towards the development of such solutions.9–13 Examples include large-scale precision monitoring of soil and weather conditions, achievement of lower detection limits, detection of previously undetectable analytes, multifunctional materials and systems for treatment of waste streams, recovery of finite resources, and efficient delivery of antibiotics, pesticides, and essential nutrients.9,14–20 Yet, the implementation of nano-enabled solutions is not without potential unintended consequences that range from increased embedded resource intensity necessary to produce the ENMs to phytotoxicity upon exposure to crops. Environmental and human health impacts are particularly germane considering the direct release and exposure pathways through their application in agriculture. The intimate connection between food systems, the environment and humans impresses vigilance in the design and development of novel applications.
Historically, exploration and growth of exciting new research areas in nanotechnology has progressed indiscriminately. Furthermore, progress is oftentimes narrowly focused on obtaining optimal performance of a material or product in the use phase; consideration of potential environmental and human health consequences is secondary rather than being incorporated as a design objective, with the goal of impact minimization. With numerous historical examples that demonstrate the ineffectiveness and potential to introduce adverse environmental and human health outcomes of the current approach,21,22 it is evident that an alternative holistic path towards development of nano-enabled agriculture is necessary.
While there are several reviews of nano-enabled applications in agriculture,9–15,17–20,23–30 this review aims to uniquely highlight the critical need for movement beyond phenomenological studies to elucidate underlying mechanisms to advance innovative and sustainable design of nano-enabled agriculture. While observation of outcomes that result from a given set of experiments provide insight into the potential for realized benefit or impact through the use of ENMs, a critical underpinning of informed design includes the mechanisms of action and specifically, the identified role of the nano-scale. This mechanistic insight is necessary to further capitalize on a beneficial outcome and to critically inform design.
Here, the current literature on nano-enabled applications in agriculture is reviewed by first identifying and compiling studies within their respective product category. The studies critically reviewed in Tables 1–5, adhere to the following tiered criteria: (i) focus on the application of an ENM or harnessing of the nano-size scale to introduce a functional performance benefit, and of those studies (ii) only those that include quantitative information about the ENM (or active ingredient) (e.g., the mass of ENM per unit nutrient or the concentration of the ENM in a given suspension) were included. In addition to being the first step towards elucidating the role of the ENM in these proposed applications, quantitative information of ENM use enables determination of the nanomaterial efficiency (i.e., how much ENM is required to achieve the desired function and/or benefit). This latter point on nanomaterial efficiency is necessary to informing design that minimizes the costs (environmental and economic) imposed through nano-enabling to achieve the added functionality or benefit. For those applications in which the nano size-scale is harnessed to realize a benefit (e.g., encapsulation of a pesticide active ingredient in a nanoemulsion), there is no physical ENM used and therefore, material efficiency gains are presented for comparisons between designs as the percent active ingredient. Nano-enabled mechanisms compiled from these studies (both suggested and empirically supported) and associated potential benefits claimed through the use of nanotechnology are also included for the reviewed studies.
| Nanomaterials used | Nanomaterial efficiencya | Nano-enabled mechanism to realize associated benefitsb | Ref. |
|---|---|---|---|
| a Presented as the mass of ENM per mass of nutrient applied (solid platforms) or the concentration of the ENM in the prepared suspension (ENM serves as the desired micronutrient applied). b Italicized text indicates the nano-enabled mechanism is proposed (i.e., empirical data is not provided to support the claim). | |||
| Hydroxyapatite (HA) NPs | 0.36–2.17 g HA NPs/g N | High surface area of HA NPs enables high binding sites for urea and demonstrates slow release of nitrogen | 59 |
| Nanoclay | 0.2–0.4 g NPs/g N | Nitrogen incorporated into self-assembled nanoclay particles form ensembles larger than soil pores enabling retention of N in soil | 57 |
| Nanostructured water-phosphorite suspension | 8.3–10.0 g NPs/g P2O5 | The nano-structure of phosphorite increases the lability of P in soil and promotes penetration into plant cells | 77 |
| Zn-loaded core–shell nanocomposite using charged polymer electrolytes | 10 g NPs/g Zn | Nano-sized manganese oxide is used as a template for a polymer electrolyte shell, which once established, is loaded with zinc sulfate; the final product is used to regulate Zn release in soils | 78 |
| Mn NPs | 0.05 ppm NPs suspension | Nano-size scale enables uptake and translocation to leaf where Mn serves as a cofactor for nitrate assimilation by stimulating the activity of enzymes (e.g., nitrate and nitrite reductase, glutamine and glutamate synthetase) | 67 |
| ZnO NPs | 0.1–1000 ppm NPs suspension | High reactivity from high specific surface area facilitates nutrient sorption leading to increased plant yield, sugar and protein content, nitrate reductase activity and antioxidant enzyme activity | 61–66 |
| Fe3O4 NPs | 1–60 ppm NPs suspension or 1 g kg−1 soil amendment | High reactivity from high specific surface area facilitates nutrient sorption | 66, 68 |
| CuNPs loaded carbon nanofiber (CuNP–CNF) | 10–500 ppm NPs suspension | CNF serves as carrier of CuNPs and controls the micronutrient (Cu) release. Small size and negatively-charged surface of CuNP–CNF enables high translocation ability into the seeds and improves osmotic condition for water uptake. High water uptake capacity then facilitates complete protein hydration and pigment synthesis | 79 |
Identifying and compiling promising nano-enabled applications across agriculture and food systems
A comprehensive literature search of nano-enabled applications being proposed for use in agriculture and food systems was conducted to identify the primary promising application categories (Fig. 1). There are two informative outcomes from this exercise: (i) there is the greatest number and diversity of potential nano-enabled applications in the in-field stage, and (ii) nano-enabling for food packaging and detection of relevant analytes (e.g., contaminants) have the greatest cross-stage applicability, ranging from the product processing to household stages.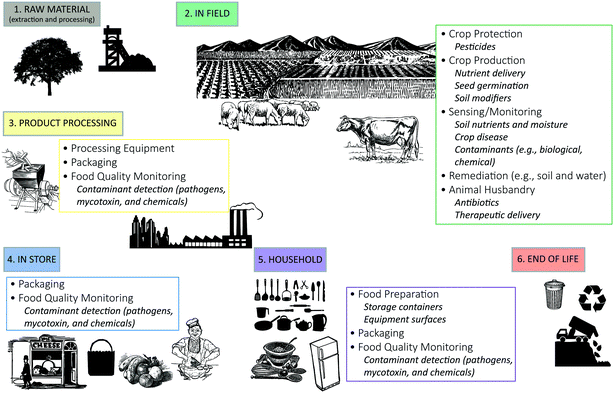 | ||
| Fig. 1 Current and proposed nano-enabled applications in agriculture and food systems identified by the life cycle stage in which they are employed. | ||
For the in-field stage, nano-enabled applications for crop cultivation practices are particularly prominent and proposed benefits realized through these applications include enhanced crop production (e.g., seed germination and enhanced biomass production25,27), improved nutrient use efficiency26 and better crop protection11 (the specific designs and nano-enabled functionality of these applications are discussed in detail in the following sections). Those nano-enabled applications having the greatest cross-stage applicability include food packaging and nano-enabled sensors for pathogen and mycotoxin detection. When crops and livestock become mature (in-field stage), nano-enabled packaging and sensing applications are being proposed for improved preservation and protection during subsequent stages (e.g., storage, food processing, transportation).
Opportunities for continued innovation: upstream processing and end-of life
In addition to these two informative outcomes, the compilation of current and proposed nano-enabled agriculture applications (Fig. 1) indicates that there are relatively few applications being considered for the materials acquisition (upstream) and end of life (downstream) stages. Understandably, many of the agriculture-related processes that are incurred during these stages are not unique to agriculture and rather, are ubiquitous across several industrial sectors. For example, many raw materials that are utilized as feedstock for agrochemicals or farming equipment are similar to those in the industrial chemicals and transportation industries. Still, one upstream application of ENMs that is under active development is the use of nano-catalysts to reduce the embedded energy associated with ammonia synthesis via the Haber–Bosch process.31–34Downstream, agricultural waste enters the same aqueous and solid waste streams as other domestic and industrial wastes, or is not captured and becomes a source of environmental pollution (e.g., eutrophication caused by excess nutrient runoff). Relatively little has been explored in terms of nano-enabled applications at these stages in general and thus, the lack of potential agriculture-specific applications elucidated here is not unique. As such, this marks an opportunity to further enhance agriculture sustainability, and research is needed to elucidate ways in which ENMs can enhance resource efficiency up- and down-stream of food production, processing and use. Some examples of potential opportunities include, monitoring and/or capturing landfill gas emissions (e.g., methane), enhancing the degradation rate and/or odor inhibition during household or commercial organic composting process, capture and recovery of primary nutrient runoff, or (pre)treatment of solid waste for resource recovery.
Nano-enabled applications that are not unique to agriculture
There are several promising ENM-enabled applications being proposed to advance capabilities and efficiencies in agriculture that build upon an established foundation of research developed for applications in other sectors, yet the underlying science and engineering remains the same. For example, applications for animal husbandry offer several promising benefits realized through the use of ENMs to reduce incidents of disease and prevent spread of infection23,35,36 as well as promote growth.37 These applications are being developed to address the projected doubling of global animal-based calorie demand by 2050,2 and leverage the vast existing body of literature on the use of nano-enabled applications for human disease prevention (e.g., vaccines), diagnosis, and treatment (e.g., therapeutic delivery).38–41 In the development of therapeutics for advancing human health, efficacy and risks are evaluated through animal testing and therefore, there is the opportunity to translate these findings to applications in animal husbandry, including eliminating therapeutic side effects, completing rapid, highly specific and sensitive diagnosis, and realizing efficient and targeted therapeutic delivery.23This same translation of findings is relevant to research pertaining to the use of ENMs for enhanced water treatment (e.g., desalination)42–46 and soil remediation (e.g., remediation of heavy metal pollution).47–49 Sources of fresh water as well as uncontaminated water and soil are critical components of safe agriculture production. While previous research in these areas may not be within the context of agriculture specifically, these same technologies can be applied to the treatment of water for irrigation and soils for crop production. Several recent studies demonstrate the utility of ENMs (e.g., TiO2 NPs, nanoscale zero-valent iron) specific to agriculture applications leveraging their high removal capacity and degradation of agrochemicals (e.g., pesticides) through photocatalytic and magnetic separation mechanisms.24,50–53
With this overview of nano-enabled applications being proposed and developed for agriculture and food systems in hand, the following section provides further insight into the identified unique application classes.
Critical review of current nano-enabled applications
In this section, the ENMs being used or proposed for use are identified and compiled within each primary application category, accompanied by the intended function and proposed benefits realized through unique properties accessed at the nano-scale. The ‘amount’ is a critical component of sustainable product design given that the ENM or nano-scale aspect of the proposed product introduces added cost, both environmental (e.g., embedded energy, materials and other resources) and economic (e.g., anticipated increase in product cost). As such, the amount of ENM or active ingredient is presented here per function provided so that the different product designs or formulations can be compared in a way that is informative for improved design that maximizes the trade-offs of costs and benefits. For those applications in which the nano size-scale rather than an ENM is leveraged (e.g., pesticides), the material efficiency is represented by the active ingredient since the goal of these applications is to enhance efficacy while reducing the total active ingredient input. Five total application classes are summarized (Tables 1–5), including nano-enabled fertilizers, crop growth regulators, pesticides, packaging and sensing of environmentally relevant analytes. In many cases, existing data gaps limited the ability to conduct the material efficiency determinations as well as the ability to extract the contribution of the ENM or nano size-scale in realizing the proposed benefit. Implications of study design and incomplete data reporting are discussed within the context of each application class, offering suggestions for ongoing research.Nano-enabled fertilizers
The development of chemical fertilizers emerged with the movement towards modern large-scale farming practices, which included the transition to continuous use of agricultural land (not allowing fallow fields to naturally restore soil nutrients). Since then, the demand for fertilizer continues to increase with an estimated 2016–17 annual global use of 192.8 Mt.54 At the same time, the amount of fertilizer required to produce one ton of grain has increased four-fold from 1970 to 2008 (from 27 kg ha−1 to 109 kg ha−1).55 In addition, nutrient use efficiency (NUE) of conventional fertilizers has remained low over the past four decades, averaging 30–35% for N, 18–20% for P, and 35–40% for K.55 Combined, low NUE and widespread use of fertilizer has led to changes in soil ecosystems (e.g., damage of soil structure, disturbance of soil microbial community), contamination of downstream aquatic systems (e.g., eutrophication), and an immense waste of embedded material and energy resources.Nano-enabled fertilizers propose to harness the nano-scale in a way that intends to deliver nutrients more efficiently and thus, reduce the impact of adverse environmental consequences that result from current inefficient fertilization practice.55 Specifically, nano-enabled fertilizers aim to increase the NUE, reduce nutrient immobilization in soil, and decrease volatilization and leaching to surrounding aquatic systems.26 ENMs are being proposed as nutrient transporters, especially for macronutrients (e.g., N, P, K), through incorporation of conventional fertilizer compounds (e.g., urea,56,57 ammonium,57 calcium phosphate,56 potassium chloride56,58) with inert nano-platforms, such as hydroxyapatite,59 zeolite,58 nanoclay,57 and chitosan56,60 NPs. In addition, ENMs can serve as a direct nutrient source, particularly for micronutrient elements (e.g., Zn, Mn, Fe), including ZnO NPs,61–66 Mn NPs,67 and iron oxide (Fe3O4) NPs.66,68–70
There are two primary mechanisms through which nano-enabling enhances NUE, (i) the nanomaterial acts as a carrier of the nutrient to be taken up by the root or plant cells via channels that are uniquely available due to the nano size-scale, and (ii) prevention of nutrient fixation and/or rapid nutrient leaching. The diameter of pores in the plant cell wall range from 5 nm to 20 nm71 such that nano-carriers smaller than the pore size enable passage through the cell wall to the cell membrane.72,73 Furthermore, interactions between the nano-carrier and plant cell can induce enlargement of pores or new pore formation,74 further facilitating uptake. Once through the cell wall, nano-carriers can be internalized through the cell membrane by endocytosis, transporter proteins, aquaporin, and ion channels.75 As a result, nutrients can be acquired by the plant cell via specific transporters, as released ions, or through the above mentioned pathways unique to nano-carriers. In addition to the diversity of uptake pathways, the interaction between a given ENM and conventional nutrients is proposed to enable slow or controlled release of the nutrients.76
Proposed formulations of nano-enabled fertilizers are compiled and reviewed in Table 1. The intended function of this product class is to provide essential nutrients for crop production. The proposed benefits realized through nano-scale phenomena include an increase in NUE, crop yield, and (micro)nutrient uptake, modified nutritional content (e.g., sugars), and the mitigation of environmental impacts caused by excess nutrient leaching. The nanomaterial efficiency is presented as the mass of ENM per unit nutrient applied.
A range of ENMs are being used as platforms for enhanced efficiency of macro- and micro-nutrient delivery to crops. For macronutrients (N, P and K), the amount of ENM per gram of nutrient ranges from 0.2–10 g/g, and for micronutrients 0.05–1000 ppm. This range in material efficiencies signals an opportunity to inform future design through choice of the platform material (for slow release fertilizer designs), and the concentration and mode of micronutrient formulation delivery. For example, to deliver Zn, the concentration of ZnO NPs ranges from 0.1 ppm65 (applied in hydroponic cultivation) to 1000 ppm63 (applied during seed germination), which indicates the potential enhanced efficiency in hydroponic systems and, given the four orders of magnitude difference, the opportunity to optimize the concentration applied during seed germination.
Still, the studies compiled in Table 1 represent a subset of studies in the current literature. Data necessary to determine material efficiency, critical to comparing designs in an informative way, precluded many studies from being included. In many of the excluded studies, the mass of the ENM and/or percent nutrient incorporation, particularly in the final formulation, was absent thus, preventing estimation of ENM per unit nutrient applied. For suspension formulations, the concentration of the ENM (serving as the micronutrient) was provided, yet the volume of suspension applied in the experiment was not reported, leaving the total quantity of ENM used to elicit the observed outcome unknown. To inform future comparison of nano-enabled fertilizer design in a way that maximizes efficiency of nanomaterial use, it is critically important for studies to include the total amount of ENM and nutrient incorporation efficiency or at minimum, the nutrient loading, and the volume and frequency of application. Finally, to evaluate the benefits of nano-enabling nutrient delivery to crops, comprehensive data collection is critical, including the total amount of nutrient applied (to confirm the reduction of nutrient input), application rates, nutrient leaching, and increased yield. Such studies should include experimental designs to answer the critical questions of whether the total amount of nutrient applied is reduced, whether the release of unused nutrients (e.g., as greenhouse gases or to water bodies) is reduced, and/or if the total yield of the final desired agriculture product (not just total biomass) increases.
Nano-enabled crop growth regulator
Growth regulators are a group of compounds, naturally occurring or synthetic, that interact with the plant hormone system to influence their metabolism and development.80 Most conventional growth regulators are plant hormones or their analogues, including auxins, ethylene, gibberellins, cytokinins, and abscisic acid.81 Recently, researchers found that ENMs can serve to promote seed germination, root elongation and crop growth, and carbon nanomaterials (CNMs) are the most widely used ENM for this application.82–89 The most prominent mechanism of CNM crop growth regulation is through the expression of aquaporin genes (e.g., LeAqp gene of tomato,90,91NtPIP1 gene of tobacco92), which regulate water uptake in plant cells. CNMs have also been shown to increase the activity of dehydrogenase, an enzyme related to reproduction and water stress response,93 which leads to enhanced water uptake.87,91 Moreover, Wang et al.87 reported increased wheat root cell length upon cultivation in medium that was amended with oxidized multi-walled carbon nanotubes (MWCNTs), which in turn facilitated biomass development. Finally, up regulation of genes involved in a plant's response to abiotic stimulus, such as TDR3 protein,90,91 heat shock protein 9090 and ripening-related mRNA,91 was observed upon exposure to CNMs. However, the connection between such gene regulation and plant growth has not yet been fully elucidated. In addition to CNMs, TiO2 NPs have also demonstrated crop growth enhancement,94,95 taking advantage of photocatalytic properties such that when exposed to sunlight, the NPs fix nitrogen from the atmosphere providing an additional source of this essential nutrient.96Proposed nano-growth regulators are compiled and reviewed in Table 2. The intended function provided by this product class is to facilitate crop development, and the proposed benefits realized through nano-scale phenomena include the promotion of seed germination, root elongation, nutrient uptake, water uptake, crop growth and adhesion of beneficial bacteria to crop roots. The nanomaterial efficiency represents the concentration of the ENM used in the formulation developed for each study.
| Nanomaterials used | Nanomaterial efficiency (ppm) | Nano-enabled mechanism to realize associated benefitsa | Ref. |
|---|---|---|---|
| a Italicized text indicates the nano-enabled mechanism is proposed (i.e., empirical data is not provided to support the claim). N/A = not available, and indicates that no nano-enabled mechanism is included in the reference and the text in parentheses indicates the description of a general mechanism discussed, but is not confirmed as being nano-specific. | |||
| Multi-wall carbon nanotubes (MWCNTs) | 10–200 | Due to their nano size, MWCNTs are able to penetrate through biological barriers (i.e., seed coat, cell wall and membrane) leading to increased water uptake, up-regulation of genes encoding aquaporin proteins and increased dehydrogenase activity | 82, 83, 87 |
| Fullerol [C60(OH)20] NPs | 0.001–0.05 | N/A | 84 |
| Water soluble carbon dots (wsCNDs) | 150 | Nano size and high surface activity enables wsCNDs to transport through the root cell barrier and carry water and nutrient into the plant | 85 |
| Water soluble carbon NPs (wsCNPs) | 50 | High adsorption capacity from functional groups and porous structure of wsCNPs enables entrapment and controlled release of nutrient | 86 |
| Water soluble carbon nano-onions (wsCNOs) | 10–30 | Porous structured wsCNOs taken up by crop via nano- and micro-scale plant openings uniformly accumulation in the xylem vessels enhances water conduction and nutrient translocation | 88 |
| Single-wall carbon nanohorns (SWCNHs) | 25–100 | N/A (SWCNHs regulate gene expression related to the stress responses and cell growth) | 89 |
| Cu NPs loaded carbon nanofiber (CuNP–CNF) | 10–500 | Small size and negatively-charged surface of CuNP–CNF enables high translocation ability into the seeds, improving osmotic condition for water uptake. High capacity for water uptake then facilitates complete protein hydration and pigment synthesis | 79 |
| TiO2 NPs | 50 | TiO 2 NP interacts with the cell surface by forming complex with the phosphate functional group on the membrane, initiating the cluster of growth promoting bacteria | 95 |
The compiled data indicate that there are many different types of CNMs being proposed to induce the desired function, including carbon nanotubes, fullerol, graphene quantum dots, water soluble carbon dots, and nano-onions. Furthermore, the amount of CNM required to elicit the desired response ranges orders of magnitude both within (e.g., 10–200 ppm for MWCNT) and between (e.g., 0.001–500 ppm) CNM types. In most studies, the CNMs were applied as a suspension during seed germination, and the relationship between exposure concentration and resulting crop growth has been identified as a critical component to evaluate the efficacy of nano-enabled growth regulators. For example, CNM concentrations below 200 ppm are generally correlated with positive effects (enhanced crop growth), while concentrations greater than 1000 ppm were found to induce phytotoxicity.97,98 Phytotoxicity is proposed to be caused by induction and accumulation of reactive oxygen species (ROS),97,99 followed by electrolyte leakage and cell death.100,101 The phytotoxic effects of CNMs are also crop species-dependent. In hydroponic culture, the root and shoot growth of red spinach and lettuce were significantly inhibited upon exposure to 1000 ppm and 2000 ppm MWCNTs, however, there were no observed negative phyto-effects for chili, lady finger, and soybean in the same exposure system.102 Furthermore, physicochemical properties of the CNMs (e.g., oxygen content, surface charge) influence their ability to regulate crop growth. For example, CNMs with high surface oxygen content carry a more negative surface charge, which has been correlated with enhanced seed germination and growth of tomato plants.103
Similar to nano-enabled fertilizers, the determination of the total quantity of CNM applied in these studies to achieve the desired outcome was hindered by the lack of reported information on the volume of CNM suspensions applied. Therefore, the concentration of the applied stock suspension is reported to compare the nanomaterial efficiency of different CNM formulations. Future reporting of the total volume of CNM suspension and frequency of application will enable the desired normalization to mass of CNM per unit function, and inform future nano-enabled growth regulator choice and design in a way that maximizes efficiency of material use. In addition, since the majority of the studies presented are based on hydroponic culture, there remains an opportunity to demonstrate the performance of proposed growth regulators in soil systems. Further, establishing dose–response trends (e.g., probing between 200 and 1000 ppm, vide supra) will enable resolution of those concentrations that result in positive effects (effective dose) and those that result in adverse effects (e.g., phytotoxicity). Finally, a common approach to synthesis of several CNMs involved hydrocarbon deposition onto a metal catalyst with compositions that include common micronutrients (e.g., copper). Comprehensive characterization of the CNM to identify and quantify the amount of residual catalyst in the sample (e.g., combined thermogravimetric analysis and elemental analysis of residual) is critical to isolating the contribution of the CNM (rather than the micronutrient) in the observed beneficial outcomes.
Nano-enabled pesticides
Over the last 50 years, pesticide use (i.e., herbicides, insecticides, fungicides) has contributed to enhanced crop productivity and improved crop quality.104 Similar to fertilizers, pesticides suffer from poor use efficiencies with only 30–40% of the applied pesticides actually reaching the target endpoint while the rest is lost to the environment.105 This introduces unintended consequences (e.g., harmful degradation products,106 loss of soil microbial communities,107 elevated human chronic disease rate108) and has motivated research to develop formulations with enhanced efficiencies.The primary approaches to ENM-enabled crop protection include loading a conventional active ingredient (e.g., atrazine,109 acetamiprid,110 methomyl111) into nano-emulsions (where the active ingredient is stabilized by a surfactant(s)) or nano-capsules (composed of polymers or lipids), using nanoparticles as the active ingredients (TiO2 NPs,112,113 AgNPs,114 nanoaluminum dust,115 nano-copper116), or using nanoparticles as carriers (e.g., mesoporous silica,117 graphene oxide118) of active ingredients. Research on the incorporation of ENMs demonstrates an increase in the stability (during storage and after application), solubility, permeability, and leaf adhesion as well as enables controlled release of active ingredients.11 Compared with their conventional counterparts, nano-enabled pesticides propose greater protection efficiency and consequently, the potential to reduce the total amount of pesticide needed for the same functional protection.109,119 Further, these applications propose the potential to enhance the ability to target a specific endpoint and thus, more effectively prevent growth of competitive weeds or invasive species, insects, pests, and fungus that compete with crops for natural resources (e.g., space, water, light, nutrients) and cause disease.120 Cytotoxicity121 and genotoxicity122 studies indicate that the ENM-modified pesticides are less toxic to the plant than the conventional formulation, suggesting an added benefit of enabling effective crop protection with reduced potential for unintended crop damage.
Several studies demonstrate the utility of nano-pesticides through enhancement of solubility and stability of the active ingredient, introduction of controlled or slow release, and reduction of premature degradation.11 Nano formulations used to increase solubility of lipophilic pesticides include nano-emulsions10 and polymer-123 or lipid-based124 nano-capsules. While detailed release profiles of formulations have not been comprehensively elucidated, a series of nano-enabled pesticides demonstrate slower release rates, which is proposed to control diffusion processes and depend on interactions between active ingredients and the compounds that constitute the stabilizing ENM structure (e.g., polymers, mesoporous structures).11,125 Certain ENMs, such as clay minerals,126 can also serve to protect the active ingredients from volatilization, degradation, and leaching,127 further increasing their environmental stability.
Proposed nano-enabled pesticides are compiled and reviewed in Table 3. The intended function of this product class is to provide crop protection and prevent damage caused by insects, fungi and weeds. The proposed benefits realized through nano-scale phenomena include enhanced solubility, permeability and stability of the active ingredient, improved adhesion to the target, controlled release, protection against premature degradation, and high injury efficiency. The compiled literature review presents material efficiency as the active ingredient or ENM concentration (as ppm) in a given formulation.
| Nanomaterials used | Active ingredient efficiency (ppm) | Nano-enabled mechanism to realize associated benefitsa | Ref. |
|---|---|---|---|
| a Italicized text indicates the nano-enabled mechanism is proposed (i.e., empirical data is not provided to support the claim). N/A = not available, and indicates that no nano-enabled mechanism is included in the reference and the text in parentheses indicates the description of a general mechanism discussed, but is not confirmed as being nano-specific. | |||
| Nano-emulsions with the following active ingredient | |||
| Glyphosate isopropylamine (IPA) | 4.1 × 105 | Lower surface tension of nano-emulsion enables retention on leaf surface with larger contact area and increased penetration of IPA | 128, 129 |
| Copaiba oil | 5.0 × 104 | N/A (nano-emulsion serves as delivery platform for copaiba oil, active ingredient, and demonstrates effective performance; no non-nano control included to identify a benefit) | 130 |
| β-Cypermethrin | 1.2 × 105 | N/A (prepared nano-emulsion shows improved stability over the commercial micro-emulsion, despite having larger droplet size) | 131 |
| Rosmarinus officinalis essential oil | 5.0 × 104 | N/A (nano-emulsion serves as delivery platform for Rosmarinus officinalis essential oil, active ingredient, and shows larvicidal activity; no non-nano control included to identify a benefit) | 132 |
| Azadirachtin | 2.0 × 105 | N/A (the synthesized amphiphilic carboxymethyl chitosan with ricinoleic acid, which is used as the emulsifier, protects azadirachtin in the micelles from degradation and leads to a controlled release pattern) | 133 |
| Nano-capsules with the following active ingredient | |||
| Atrazine | 106 | The hydrophobic property of the nanocapsules enables their interaction with leaf cuticle, leading to increased atrazine delivery into plant and reduced herbicide loss to the environment | 109 |
| Methomyl | 50–100 | Methomyl is encapsulated by forming hydrogen bonding with carboxylic groups on the inner surface of the nanocapsules shell at pH 4.0, which are ionized at pH 6.0, resulting in the destruction of hydrogen bonding and providing a diffusion controlled release of methomyl | 111 |
| Acetamiprid | 9.7 × 104 | Nano-capsules act as physical barriers for the encapsulated pesticide | 110 |
| Mancozeb | 1.9 × 105 | Nano-formulations provide a protective barrier for the entrapped pesticide | 134 |
| Nanomaterials | Nanomaterial efficiency (ppm) | ||
| Straw ash-based biochar and biosilica (BCS) | 2000–4800 | High porosity and specific surface area of BCS enables high adsorption capacity of active ingredient and contributes to controlled release | 117 |
| Zinc doped nTiO2 | 500–800 | Photocatalytic property of nTiO2 enables microbial inactivation by creating reactive oxygen species under illumination. Smaller size of nTiO2 possesses an enhanced antibacterial activity due to increased specific surface area. Additionally, Zn contained in nTiO2 shifts its absorbance spectrum to a region with doubled solar energy flux, which also contributes to the improved antibacterial efficacy | 112, 113 |
| AgNPs | 10–100 | Small size and high specific surface area of AgNPs increase their contact with microbial cells and the antifungal properties might be realized through compromising membrane integrity | 114 |
| Nanoalumina dust | 125–250 | The smaller size of nano-alumina dust enables stronger absorptivity for epicuticular hydrocarbons via capillary forces, thus enhancing insecticidal activity | 115 |
| Nano-copper (nCu) | 0.2–20 | Electrostatic interactions promote adhesion of nCu to cell wall initiating redox reactions | 116 |
The nano-enabled pesticide literature could be greatly enhanced through identification of mechanisms through which the nano-scale facilitates the observed benefits, as demonstrated through the presentation of study outcomes in the third column of Table 3. In many studies, the mechanism is either proposed or not available, which in both cases, indicates the lack of empirical evidence to inform ongoing research for improved product design. Another important outcome identified from reviewing the literature is the critical need for including non-nano controls in the experimental design to compare and confirm the enhanced active ingredient efficiency. Finally, while the comparative efficacy and novel functionality that accompanies the incorporation of nano-scale design of pesticides are demonstrated, there remains an important need to comprehensively assess the environmental benefits of nano-enabled pesticides (i.e., the reduced impacts via enhanced active ingredient efficiency) while also considering the potential risks posed by the nano-carrier.135 The proposed benefits through nano-enabling to date, rely upon laboratory studies designed to demonstrate the comparative efficacy. Given the large-scale application of pesticides and range of environmental conditions they will experience, additional laboratory-based short- and long-term studies as well as larger-scale (simulated) field studies are needed to truly evaluate these trade-offs.
Nano-enabled packaging
Most agriculture and food products are packaged post production. The purpose of packaging is to hold the contents (e.g., liquids such as milk or juice), as well as protect these products during transport and preserve their freshness during storage (e.g., meat, produce). Packaging provides multiple levels of protection, including against physical damage and contamination (e.g., biological, chemical), and utilizes a range of packaging materials (e.g., glass, plastic, coated paperboard), which are chosen based on the contents and intended shelf life.136,137 Still, existing limitations, including occurrences of product damage, food spoilage, harnessing and (un)intentional introduction of harmful contaminants,138–140 have motivated a movement towards development of enhanced packaging materials.The incorporation of ENMs is being pursued as a way to enhance packaging properties (e.g., enhanced barrier to atmospheric gases and water vapor, more robust materials to prevent compromised packaging) for improved protection of agriculture and food system products. Further, their incorporation can introduce novel functionality, such as the ability to detect spoilage and contamination.141–143 In 2015, the nano-enabled packaging market was $23.73 billion and is anticipated to experience continued growth due to the market's identified potential.144
Ag NPs, ZnO NPs, nanoclay, TiO2 NPs, and nano-starch are being incorporated into packaging materials to serve as antimicrobial agents (e.g., inactivation of food-borne pathogens), moisture barriers (e.g., preserve freshness), and/or material strength enhancers (e.g., prevent compromising protective packaging).30,141,142,145 In addition to preservation and protection, ENMs are being used in food preparation products, including the incorporation or coating of plastic food containers with nano-ceramic, TiO2 NPs, and ZnO NPs to resist deterioration, inhibit odor generation, and enhance the aesthetic appeal.146 The photocatalytic property of these ENMs adds self-cleaning and disinfectant properties to the plastics, providing an opportunity to reduce the use of detergents for cleaning.146
Proposed nano-enabled food packaging designs are compiled in Table 4. The function of this application class is to protect food and agriculture products. The proposed benefits realized through nano-scale phenomenon include improved mechanical properties and the ability to provide a physical barrier, thermal barrier, antimicrobial activity, and protection from UV exposure (Note: The incorporation of ENMs to detect contaminants is reviewed in the nano-enabled sensors section that follows). Information about the nanomaterial efficiency is presented as a loading, the mass of ENMs, based on the data available data in the literature.
| Nanomaterials used | Nanomaterial efficiency (ppm) | Nano-enabled mechanism to realize associated benefitsa | Ref. |
|---|---|---|---|
| a Italicized text indicates the nano-enabled mechanism is proposed (i.e., empirical data is not provided to support the claim). | |||
| AgNPs | 250–2000 | • Incorporation of AgNPs reduces the free volume and creates a tortuous path that prevents passage of water vapor and oxygen | 147–150 |
| • Large specific surface area of AgNP facilitates the release of Ag ions, which react with the biomolecules containing sulfur, nitrogen or oxygen in bacterial cells, causing loss of viability | |||
| Nanoclay | 1.0 × 104–1.0 × 105 | • Strong interfacial interaction between the packaging polymer and nanoclay restricts mobility of polymer chains, increases the tortuosity of the film, and fills in voids to suppress heat and oxygen transfer | 151–159 |
| • Quaternary ammonium groups in the silicate layer of nanoclay disrupt the bacterial cell membrane, leading to cell lysis | |||
| ZnO NPs | 500–1.0 × 105 | • High surface energy and large specific surface area of ZnO NPs give rise to strong interactions with the packaging polymer molecules, leading to the reduced mobility and increased interactive force of polymer chains as well as creation of a tortuous path to prevent passage of water vapor | 160–167 |
| • Inherent high thermal conductivity and mass transfer barrier properties of ZnO NPs retard thermal degradation of films by facilitating heat dissipation while inhibiting the transport of generated volatile products during decomposition | |||
| • ZnO NPs can damage cell membrane by mediating hydrogen peroxide production, cause mechanical damage of bacteria via penetration, and release Zn ions to interact with interior components | |||
| TiO2 NPs | 2000–4.8 × 104 | • Photocatalytic properties of TiO2 NPs enable strong interfacial interaction with polymer molecules, resulting in the formation of a stable three-dimensional polymeric matrix | 168–173 |
| • Low hydrophilicity of TiO2 NPs and high compatibility with polymer molecules prevent water absorption and increase circuitous routes for water molecules to pass through the film, respectively | |||
| • High thermal stability of TiO2 NPs | |||
| • Photocatalytic properties of TiO2NPs enable production of strong oxidizing species under UV illumination | |||
| • High UV light absorption of TiO2 NPs | |||
| CNTs | 5000–7 × 104 | • Strong interfacial interactions between CNTs and the packaging polymer enables homogeneous dispersion in the polymer matrix. High inherent elastic modulus and tensile strength of CNTs are introduced to the packaging film | 174–176 |
| • A tortuous path is created through incorporation of CNTs inhibiting the diffusion of water vapor and oxygen molecules | |||
| • High thermal stability of CNTs | |||
| • CNTs can interact with and physically damage bacterial | |||
| • Cell membranes, causing irreversible cell damage | |||
| Starch nanocrystals (SNCs) | 1 × 104–5 × 104 | • Dense structure and high stiffness of SNCs compared with starch matrix | 177 |
| • Well-dispersed SNCs increased the compatibility of the film and introduced a tortuous path for water transfer | |||
| • Interaction between SNCs and starch chains increased the crystallinity of the film | |||
Again, a wide range of ENMs, including AgNPs, nanoclay, ZnO NPs, TiO2 NPs, and CNTs, are being proposed and incorporated into polymer matrices to realize the desired benefits of nano-enabled food packaging. The primary role of the ENM is to enhance the barrier against water vapor and oxygen as well as impart antimicrobial properties against food-borne bacteria, both of which lead to an extended food lifetime. In addition, there is the opportunity to leverage multiple properties and benefits from a particular ENM. For example, the thermal stability and photocatalytic property of TiO2 provides multiple mechanisms of protection to prevent exposure induced spoilage.169
In many of the compiled studies, the properties of packaging can be significantly improved with the addition of a low quantities of the ENMs. Since the addition of ENMs into packaging materials introduces added complexity and potential exposure routes, minimizing the concentration of ENM per realized benefit is important from an upstream embedded energy, downstream handling, and economic perspective. Further, while recycling rates have increased over time (34.6% in 2014, up from 16.0% in 1990), packaging still remains the largest portion of municipal solid waste (29.5%, 76 million tons).178 Thus, there is an opportunity to consider how the incorporation of ENMs could enhance the potential for packaging recycling, introducing an added system benefit.
Nano-enabled sensing for detection of agriculturally relevant analytes
While the nano-enabled sensor literature is vast, those specifically targeting the agriculture and food systems sector are reviewed here. The growing demand for site-specific crop management (or precision agriculture) as a way to reduce economic and environmental burden of crop production has motivated the development and application of nano-enabled sensors to obtain real-time and comprehensive field condition information (e.g., soil moisture or nutrient levels). Furthermore, the application of nano-enabled sensors is proposed as an effective approach to ensure food safety and reduce the incidence of food-borne illness.141,179 Consumers generally judge the quality of packaged food based on the indicated sell-by or expiration dates, however these may not reflect the actual state of food quality. As such, ENMs offer a path towards real-time monitoring of pathogens, mycotoxins, and residual chemicals (e.g., pesticides) to inform consumers of food quality. Furthermore, real-time food quality monitoring has the potential to significantly reduce the 40% of all US-produced food that is wasted and produces 133 billion pounds of food waste annually.180 Finally, an exciting frontier of ENM-enabled sensing includes the incorporation of CNMs in plants for the detection of environmentally relevant analytes (e.g., nitroaromatic compounds and nitric oxide).181,182 In this way, the plant becomes the sensor, providing a way to detect otherwise undetectable analytes (or those that are extremely challenging to monitor).By exploiting the novel properties of ENMs (e.g., surface plasma resonance, photoactivity), nano-enabled sensors have advanced to overcome deficiencies of conventional sensors, exhibiting enhanced sensitivity, selectivity, reliability, reproducibility and portability.12,183 Consequently, ENM-enabled sensor systems present biological, electrochemical and optical mechanisms for the detection of heavy metals, pesticides, pathogens, and toxins.13,184 It is important to note that the nano-enabled sensors reviewed here are lab-scale prototypes and/or intended for primary use through direct exposure of the ENM (typically in suspension) to an environmental sample. As such, there is no physical platform or casing that one may conjure of a typical sensor platform or device.
Proposed nano-enabled sensing applications in agriculture are compiled in Table 5, and include applications for detection of analytes in soil, water and food packaging. The intended function provided by this product class is the detection of a target analyte. The proposed benefits realized through nano-scale phenomena include enhanced sensitivity and selectivity, portability and remote sensing. Slightly different in format to the previous tables, the designs for sensing are organized around the target analyte. Since there are several ENMs that emerge in multiple analyte categories, the nano-enabled mechanisms to realize associated benefits column is organized by ENM. The prevailing ENMs integrated into the nanosensors include metals, such as AuNPs and AgNPs,185–193 CNMs,194–198 magnetic composites199,200 and quantum dots,201,202 with colorimetric and electrochemical primary mechanisms of detection.12 The unit of material efficiency used to compare the different ENM-enabled sensors is presented as the mass (ng) required per detection event.
| Nanomaterials used | Nanomaterial efficiency (ng per measurement) | Nano-enabled mechanism to realize associated benefits | Ref.a |
|---|---|---|---|
| a References refer to studies conducted for the ENM listed in the same row. | |||
| Water/soil monitoring (e.g., heavy metal detection) | |||
| AuNPs | |||
| AuNPs | 0.0099–980 | • Tunable surface plasmon resonance (SPR) enables the size-dependent optical properties for colorimetric sensors | 185–189 |
| • Effective quenching of chromophores through energy- and electron-transfer process for the nanomaterial surface energy transfer (NSET) probes | 201 | ||
| CdTe quantum dots | 530 | • Functionalized AuNPs can provide biocompatibility, high stability, and tunability of the surface interactions with recognition elements (e.g., enzyme, nucleic acids) and target analytes | |
| Pesticide detection | |||
| CdTe quantum dots | |||
| AuNPs | 5.5 | • Superior to the conventional organic fluorescent dyes due to high signal intensity and wide excitation spectra | 190 |
| AgNPs | 7200 | • Tunable SPR enables the size-dependent optical properties for colorimetric sensors | 191 |
| Fe3O4 | 5.0 × 104 | • Strong affinity to target analytes (e.g., some pesticides) via covalent interaction for chemiluminescent sensor arrays | 199 |
| Pathogen detection | |||
| Magnetic nanoparticles (MNPs)-Fe3O4 and FeCl3 | |||
| AuNPs | 3.1 | • Superparamagnetic behavior for efficient capture and immunomagnetic preconcentration of pathogens | 192 |
| FeCl3 | 9.7 × 104 | • Peroxidase activity to effectively catalyze the oxidation of colorimetric substrates, triggering the color reaction | 203 |
| Fe3O4 | 2000–2.5 × 106 | 200, 204 | |
| nTiO2 | |||
| TiO2 | 2000 | • Stable absorption spectra serving as an optical nanoprobe over a wide range of pH, temperature, and salt concentrations | 204 |
| Mycotoxin detection | |||
| Carbon nanomaterials (CNMs)-graphene oxides and carbon nanotubes | |||
| AuNPs | 0.14 | • Promote strong electrostatic interaction with the molecular recognition agents (e.g., dye-labeled aptamers) in the biosensors | 193 |
| Graphene oxides | 4800 | 194 | |
| Carbon nanotubes | 2.0 × 104 | 195 | |
The nano-sensor literature is the most established reviewed herein, and research on the unique properties of different ENMs that are leveraged for detection is well-established. As such, the mechanisms enabled through the incorporation of ENMs to realize the associated benefits are empirically well-supported. Still, similar to all other product categories reviewed, there is a diverse range in ENMs that can be used to detect a given analyte. For example, metals, metal oxides, CNMs and quantum dots can all be used to detect pesticides.190,191,199,202,205–208 The range in amount of ENM necessary to elicit a detection event, both within and between a given ENM class also varies significantly, signaling the opportunity to optimize efficiency of ENM use through informed material selection in a given sensor design. For example, within the category of pesticide detection, the amount of Au and Fe3O4 NPs differs by four orders of magnitude, indicating the enhanced sensitivity of Au for the target pesticide analyte. Different sensor designs that utilize AuNPs for soil monitoring range five orders of magnitude per detection event. Finally, the same ENM can be used to target multiple analytes, which suggests the potential to consider design of multi-functional sensing devices.
Limited information on the amount of ENM used in a given design prevented inclusion of all proposed nano-enabled sensor designs for agriculture applications found in the literature. For example, ENMs used in food packaging to detect parameters such as humidity or a specific indicator molecule (e.g., ethanol, ethylene), do not report the quantity of ENM incorporated into the packaging material.209 CNMs used in modified electrodes for electrochemical detection may report the concentration of the CNM suspension, but not the volume loaded onto the electrode.207,208,210 As such, data availability limits the ability to formulate meaningful comparisons of all proposed nano-enabled sensors in the literature.
Suggestions for ongoing research to inform sustainable nano-enabled agriculture design
Current research on the use of ENMs and harnessing of the nano size-scale in a wide range of agriculture-related applications demonstrates the opportunity to introduce added functionality, improve performance, and/or enhance efficiency over current practice and technologies, all of which aim to advance agriculture sustainability. The mechanisms through which ‘nano’ contributes to this realization have been identified, when possible, in Tables 1–5. It is this mechanistic insight that is critical to enabling continued innovation in product design to further enhance the desired outcome; without it, we are left with an indiscriminate approach to design based on phenomenological results and limited advancement towards design for optimized performance.To encourage rapid advancement and innovative design in a way that promotes agriculture sustainability, three informative guidelines identified from this literature review are described to inform ongoing studies:
1. Comprehensive data reporting, specifically as it relates to the ENM or active ingredient used, is critical and should include:
a. characterization of key nano-scale properties,
b. the mass of ENM in the product or formulation, or the incorporation efficiency (of the ENM or active ingredient), whichever is appropriate for the given product class, and
c. the volume or amount and frequency of application.
When possible or applicable, include tracking of the material through the system (i.e., provide information necessary to complete an ENM mass balance).
2. The design of experiments should include non-nano controls needed to identify and quantify the benefit provided by the nano-enabled product, if any.
3. The mechanism (proposed and when possible, empirically supported) underlying how the ‘nano’ aspect of the design is enabling the observed outcome should be identified and discussed, as this information is critical to (i) confirming that the nano-aspect, rather than a non-nano artifact, of the design governs the desired outcome, and (ii) informing continued sustainable design of promising solutions.
In addition to this pointed guidance for ongoing studies, the research community should consider establishing a suggested minimum set of standard testing guidelines to include, (i) a concentration range and crop type(s) used for exposure studies, and (ii) metrics for benefit quantification (e.g., root and shoot length, yield as dry biomass or the desired product, nutritional content) for application studies. This will facilitate, (i) comparison of results between studies, (ii) material efficiency optimization, and (iii) informed design improvements to maximize functional efficacy. This information is necessary to quantify trade-offs of nano-enabling agriculture early in the design process in an effort to preclude future unintended consequences.
Finally, economics is a critical criterion to consider in the development of any new product or approach to be applied to agriculture and food systems. New technologies will only be realized if the economics is advantageous for both the consumer and the producer, both of whom are driven by cost minimization. Considering the economics of the system, not just the cost of the product, is critical to nanotechnologies in particular, since the product may demand higher capital cost to realize a net positive revenue stream (e.g., introduce cost savings further downstream via reduction of agrochemical use and/or enhanced crop yield). As with any emerging technology, the novelty of nano-enabled applications in agriculture as well as their predominantly lab-scale production drive high and uncertain cost. Synthetic pathways for some ENMs are in relative infancy, with ongoing efforts to reduce resource intensity and increase yields. High initial costs in conjunction with high levels of uncertainty surrounding wide-spread implementation will remain primary obstacles to adoption, yet established uncertainty and techno economic approaches in conjunction with life cycle assessment can provide guidance for early decision-making in the product design to minimize cost and environmental impact.211,212
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the generous funding support from the Department of Civil and Environmental Engineering in the Swanson School of Engineering at the University of Pittsburgh and Leila Pourzahedi for her assistance in creating the TOC art.References
- D. Tilman, C. Balzer, J. Hill and B. L. Befort, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20260–20264 CrossRef CAS PubMed.
- B. L. Bodirsky, S. Rolinski, A. Biewald, I. Weindl, A. Popp and H. Lotze-Campen, PLoS One, 2015, 10, e0139201 Search PubMed.
- UN DESA, World Population Prospects: Key findings and advance tables, 2015 Revision, UN DESA, New York, NY, 2015 Search PubMed.
- D. Tilman, K. G. Cassman, P. A. Matson, R. Naylor and S. Polasky, Nature, 2002, 418, 671 CrossRef CAS PubMed.
- N. D. Mueller, J. S. Gerber, M. Johnston, D. K. Ray, N. Ramankutty and J. A. Foley, Nature, 2012, 490, 254 CrossRef CAS PubMed.
- S. Dall'erba and F. Domínguez, Spat. Econ. Anal., 2016, 11, 46–66 CrossRef.
- S. Piao, P. Ciais, Y. Huang, Z. Shen, S. Peng, J. Li, L. Zhou, H. Liu, Y. Ma and Y. Ding, Nature, 2010, 467, 43–51 CrossRef CAS PubMed.
- C. Rosenzweig, A. Iglesias, X. Yang, P. R. Epstein and E. Chivian, Global Change and Human Health, 2001, 2, 90–104 CrossRef.
- S. M. Rodrigues, P. Demokritou, N. Dokoozlian, C. O. Hendren, B. Karn, M. S. Mauter, O. A. Sadik, M. Safarpour, J. M. Unrine, J. Viers, P. Welle, J. C. White, M. R. Wiesner and G. V. Lowry, Environ. Sci.: Nano, 2017, 4, 767–781 RSC.
- L. R. Khot, S. Sankaran, J. M. Maja, R. Ehsani and E. W. Schuster, Crop Prot., 2012, 35, 64–70 CrossRef CAS.
- M. Nuruzzaman, M. M. Rahman, Y. Liu and R. Naidu, J. Agric. Food Chem., 2016, 64, 1447–1483 CrossRef CAS PubMed.
- G. Bülbül, A. Hayat and S. Andreescu, Sensors, 2015, 15, 30736–30758 CrossRef PubMed.
- S. Baruah and J. Dutta, Environ. Chem. Lett., 2009, 7, 191–204 CrossRef CAS.
- H. Chen and R. Yada, Trends Food Sci. Technol., 2011, 22, 585–594 CrossRef CAS.
- C. Parisi, M. Vigani and E. R. Cerezo, Proceedings of a Workshop on “Nanotechnology for the agricultural sector: from research to the field”, IDEAS, 2014 Search PubMed.
- N. Sozer and J. L. Kokini, Trends Biotechnol., 2009, 27, 82–89 CrossRef CAS PubMed.
- P. Wang, E. Lombi, F. J. Zhao and P. M. Kopittke, Trends Plant Sci., 2016, 21, 699–712 CrossRef CAS PubMed.
- C. R. Kagan, ACS Nano, 2016, 10, 2985–2986 CrossRef CAS PubMed.
- N. Arif, V. Yadav, S. Singh, S. Singh, R. K. Mishra, S. Sharma, N. Dubey, D. Kumar Tripathi and D. Chauhan, J. Environ. Anal. Toxicol., 2016, 6, 1–5 Search PubMed.
- H. Chen, J. N. Seiber and M. Hotze, J. Agric. Food Chem., 2014, 62, 1209–1212 CrossRef CAS PubMed.
- L. M. Gilbertson, J. B. Zimmerman, D. L. Plata, J. E. Hutchison and P. T. Anastas, Chem. Soc. Rev., 2015, 44, 5758–5777 RSC.
- J. B. Zimmerman and P. T. Anastas, Science, 2015, 347, 1198–1199 CrossRef CAS PubMed.
- A. Manuja, B. Kumar and R. K. Singh, Nanotechnol. Dev., 2012, 2, 4 CrossRef.
- G. Aragay, F. Pino and A. Merkoci, Chem. Rev., 2012, 112, 5317–5338 CrossRef CAS PubMed.
- A. Husen and K. S. Siddiqi, J. Nanobiotechnol., 2014, 12, 16 CrossRef PubMed.
- R. Liu and R. Lal, Sci. Total Environ., 2015, 514, 131–139 CrossRef CAS PubMed.
- A. Mukherjee, S. Majumdar, A. D. Servin, L. Pagano, O. P. Dhankher and J. C. White, Front. Plant Sci., 2016, 7, 1–16 Search PubMed.
- N. Dasgupta, S. Ranjan and C. Ramalingam, Environ. Chem. Lett., 2017, 15, 591–605 CrossRef CAS.
- R. Prasad, A. Bhattacharyya and Q. D. Nguyen, Front. Microbiol., 2017, 8, 1–13 Search PubMed.
- N. Bumbudsanpharoke, J. Choi and S. Ko, J. Nanosci. Nanotechnol., 2015, 15, 6357–6372 CrossRef CAS PubMed.
- S. Licht, B. Cui, B. Wang, F.-F. Li, J. Lau and S. Liu, Science, 2014, 345, 637–640 CrossRef CAS PubMed.
- Y. Lu, Y. Yang, T. Zhang, Z. Ge, H. Chang, P. Xiao, Y. Xie, L. Hua, Q. Li and H. Li, ACS Nano, 2016, 10, 10507–10515 CrossRef CAS PubMed.
- J. M. P. Martirez and E. A. Carter, ACS Nano, 2016, 10, 2940–2949 CrossRef CAS PubMed.
- J. M. P. Martirez and E. A. Carter, J. Am. Chem. Soc., 2017, 139, 4390–4398 CrossRef CAS PubMed.
- Y. Shi, Z. Xu, J. Feng and C. Wang, Anim. Feed Sci. Technol., 2006, 129, 138–148 CrossRef CAS.
- Y. H. Shi, Z. R. Xu, C. Z. Wang and Y. Sun, N. Z. J. Agric. Res., 2007, 50, 473–478 CrossRef CAS.
- T. Guo, Y. Ma and Z. Xu, Zhongguo Xumu Zazhi, 2007, 21, 007 Search PubMed.
- Z. Lu and S. Sakuma, Nanomaterials in Pharmacology, Humana Press, 2016 Search PubMed.
- J. A. Hubbell and A. Chilkoti, Science, 2012, 337, 303–305 CrossRef PubMed.
- J. H. Adair, M. P. Parette, E. I. Altinoglu and M. Kester, ACS Nano, 2010, 4, 4967–4970 CrossRef CAS PubMed.
- S. Mitragotri, D. G. Anderson, X. Y. Chen, E. K. Chow, D. Ho, A. V. Kabanov, J. M. Karp, K. Kataoka, C. A. Mirkin, S. H. Petrosko, J. J. Shi, M. M. Stevens, S. H. Sun, S. Teoh, S. S. Venkatraman, Y. N. Xia, S. T. Wang, Z. Gu and C. J. Xu, ACS Nano, 2015, 9, 6644–6654 CrossRef CAS PubMed.
- X. Qu, P. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931–3946 CrossRef CAS PubMed.
- R. Das, M. E. Ali, S. B. A. Hamid, S. Ramakrishna and Z. Z. Chowdhury, Desalination, 2014, 336, 97–109 CrossRef CAS.
- J. R. Werber, C. O. Osuji and M. Elimelech, Nat. Rev. Mater., 2016, 1, 16018 CrossRef CAS.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. Alvarez, Water Res., 2008, 42, 4591–4602 CrossRef CAS PubMed.
- P. Westerhoff, P. Alvarez, Q. Li, J. Gardea-Torresdey and J. Zimmerman, Environ. Sci.: Nano, 2016, 3, 1241–1253 RSC.
- M. M. Rabbani, I. Ahmed and S. J. Park, in Environmental remediation technologies for metal-contaminated soils, Springer, 2016, pp. 219–229 Search PubMed.
- B. Karn, T. Kuiken and M. Otto, Environ. Health Perspect., 2009, 1823–1831 Search PubMed.
- A. B. Cundy, L. Hopkinson and R. L. Whitby, Sci. Total Environ., 2008, 400, 42–51 CrossRef CAS PubMed.
- N. M. Mahmoodi and M. Arami, J. Alloys Compd., 2010, 506, 155–159 CrossRef CAS.
- A. Hu, X. Zhang, K. D. Oakes, P. Peng, Y. N. Zhou and M. R. Servos, J. Hazard. Mater., 2011, 189, 278–285 CrossRef CAS PubMed.
- H. Chen, S. Yang, K. Yu, Y. Ju and C. Sun, J. Phys. Chem. A, 2011, 115, 3034–3041 CrossRef CAS PubMed.
- Y. Zhang, Y. Li and X. Zheng, Sci. Total Environ., 2011, 409, 625–630 CrossRef CAS PubMed.
- P. Heffer and M. Prud'homme, Doha, Qatar, 2012.
- K. S. Subramanian, A. Manikandan, M. Thirunavukkarasu and C. S. Rahale, in Nanotechnol. Food Agr., Springer, 2015, pp. 69–80 Search PubMed.
- E. Corradini, M. De Moura and L. Mattoso, eXPRESS Polym. Lett., 2010, 4, 509–515 CrossRef CAS.
- D. Cai, Z. Wu, J. Jiang, Y. Wu, H. Feng, I. G. Brown, P. K. Chu and Z. Yu, Sci. Rep., 2014, 4, 1–8 Search PubMed.
- N. Zwingmann, I. D. Mackinnon and R. J. Gilkes, Appl. Clay Sci., 2011, 53, 684–690 CrossRef CAS.
- N. Kottegoda, C. Sandaruwan, G. Priyadarshana, A. Siriwardhana, U. Rathnayake, D. Berugoda Arachchige, A. Kumarasinghe, D. Dahanayake, V. Karunaratne and G. Amaratunga, ACS Nano, 2017, 11, 1214–1221 CrossRef CAS PubMed.
- H. M. Abdel-Aziz, M. N. Hasaneen and A. M. Omer, Span. J. Agric. Res., 2016, 14, 0902 Search PubMed.
- R. Raliya and J. Tarafdar, Agric. Res., 2013, 2, 48–57 CrossRef CAS.
- L. Zhao, Y. Sun, J. A. Hernandez-Viezcas, A. D. Servin, J. Hong, G. Niu, J. R. Peralta-Videa, M. Duarte-Gardea and J. L. Gardea-Torresdey, J. Agric. Food Chem., 2013, 61, 11945–11951 CrossRef CAS PubMed.
- T. Prasad, P. Sudhakar, Y. Sreenivasulu, P. Latha, V. Munaswamy, K. R. Reddy, T. Sreeprasad, P. Sajanlal and T. Pradeep, J. Plant Nutr., 2012, 35, 905–927 CrossRef CAS.
- M. A. El-Kereti, S. A. El-feky, M. S. Khater, Y. A. Osman and E.-S. A. El-sherbini, Recent Pat. Food, Nutr. Agric., 2013, 5, 169–181 CrossRef CAS.
- N. Singh, N. Amist, K. Yadav, D. Singh, J. Pandey and S. Singh, J. Nanoeng. Nanomanuf., 2013, 3, 353–364 CrossRef CAS.
- A. S. Soliman, S. A. El-feky and E. Darwish, J. Hortic. For., 2015, 7, 36–47 CrossRef.
- S. Pradhan, P. Patra, S. Mitra, K. K. Dey, S. Jain, S. Sarkar, S. Roy, P. Palit and A. Goswami, J. Agric. Food Chem., 2014, 62, 8777–8785 CrossRef CAS PubMed.
- M. Shahrekizad, A. Gholamalizadeh Ahangar and N. Mir, J. Nanostruct., 2015, 5, 117–127 Search PubMed.
- J. Li, J. Hu, L. Xiao, Q. Gan and Y. Wang, Water, Air, Soil Pollut., 2017, 228, 52 CrossRef.
- P. Das, K. Sarmah, N. Hussain, S. Pratihar, S. Das, P. Bhattacharyya, S. A. Patil, H.-S. Kim, M. I. A. Khazi and S. S. Bhattacharya, RSC Adv., 2016, 6, 103012–103025 RSC.
- A. Fleischer, M. A. O'Neill and R. Ehwald, Plant Physiol., 1999, 121, 829–838 CrossRef CAS PubMed.
- M. Moore, Environ. Int., 2006, 32, 967–976 CrossRef CAS PubMed.
- E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser, A. J. Miao, A. Quigg, P. H. Santschi and L. Sigg, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS PubMed.
- M. Khodakovskaya, E. Dervishi, M. Mahmood, Y. Xu, Z. Li, F. Watanabe and A. S. Biris, ACS Nano, 2009, 3, 3221–3227 CrossRef CAS PubMed.
- R. Nair, S. H. Varghese, B. G. Nair, T. Maekawa, Y. Yoshida and D. S. Kumar, Plant Sci., 2010, 179, 154–163 CrossRef CAS.
- H. Wanyika, E. Gatebe, P. Kioni, Z. Tang and Y. Gao, J. Nanosci. Nanotechnol., 2012, 12, 2221–2228 CrossRef CAS PubMed.
- N. Sharonova, A. K. Yapparov, N. S. Khisamutdinov, A. Ezhkova, I. Yapparov, V. Ezhkov, I. Degtyareva and E. Babynin, Nanotechnol. Russ., 2015, 10, 651–661 CrossRef CAS.
- M. Yuvaraj and K. Subramanian, Soil Sci. Plant Nutr., 2015, 61, 319–326 CrossRef CAS.
- M. Ashfaq, N. Verma and S. Khan, Environ. Sci.: Nano, 2016, 4, 138–148 RSC.
- W. Rademacher, J. Plant Growth Regul., 2015, 34, 845–872 CrossRef CAS.
- H. Kende and J. Zeevaart, Plant Cell, 1997, 9, 1197 CrossRef CAS PubMed.
- D. Tiwari, N. Dasgupta-Schubert, L. V. Cendejas, J. Villegas, L. C. Montoya and S. B. Garcia, Appl. Nanosci., 2014, 4, 577–591 CrossRef CAS.
- M. H. Lahiani, E. Dervishi, J. Chen, Z. Nima, A. Gaume, A. S. Biris and M. V. Khodakovskaya, ACS Appl. Mater. Interfaces, 2013, 5, 7965–7973 CAS.
- C. Kole, P. Kole, K. M. Randunu, P. Choudhary, R. Podila, P. C. Ke, A. M. Rao and R. K. Marcus, BMC Biotechnol., 2013, 13, 1 CrossRef PubMed.
- S. Tripathi and S. Sarkar, Appl. Nanosci., 2015, 5, 609–616 CrossRef CAS.
- M. Saxena, S. Maity and S. Sarkar, RSC Adv., 2014, 4, 39948–39954 RSC.
- X. Wang, H. Han, X. Liu, X. Gu, K. Chen and D. Lu, J. Nanopart. Res., 2012, 14, 1–10 Search PubMed.
- S. K. Sonkar, M. Roy, D. G. Babar and S. Sarkar, Nanoscale, 2012, 4, 7670–7675 RSC.
- M. H. Lahiani, J. Chen, F. Irin, A. A. Puretzky, M. J. Green and M. V. Khodakovskaya, Carbon, 2015, 81, 607–619 CrossRef CAS.
- M. V. Khodakovskaya, K. de Silva, D. A. Nedosekin, E. Dervishi, A. S. Biris, E. V. Shashkov, E. I. Galanzha and V. Zharov, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 1028–1033 CrossRef CAS PubMed.
- M. H. Lahiani, E. Dervishi, I. Ivanov, J. Chen and M. Khodakovskaya, Nanotechnology, 2016, 27, 265102 CrossRef PubMed.
- M. V. Khodakovskaya, K. de Silva, A. S. Biris, E. Dervishi and H. Villagarcia, ACS Nano, 2012, 6, 2128–2135 CrossRef CAS PubMed.
- Y. Yoshiba, T. Kiyosue, K. Nakashima, K. Yamaguchi-Shinozaki and K. Shinozaki, Plant Cell Physiol., 1997, 38, 1095–1102 CrossRef CAS PubMed.
- R. Rafique, M. Arshad, M. Khokhar, I. Qazi, A. Hamza and N. Virk, NUST J. Eng. Sci., 2015, 7, 42–46 Search PubMed.
- N. Palmqvist, S. Bejai, J. Meijer, G. Seisenbaeva and V. Kessler, Sci. Rep., 2015, 5, 10146 CrossRef CAS PubMed.
- F. Yang, C. Liu, F. Gao, M. Su, X. Wu, L. Zheng, F. Hong and P. Yang, Biol. Trace Elem. Res., 2007, 119, 77–88 CrossRef CAS PubMed.
- P. Begum and B. Fugetsu, J. Hazard. Mater., 2012, 243, 212–222 CrossRef CAS PubMed.
- J. E. Cañas, M. Long, S. Nations, R. Vadan, L. Dai, M. Luo, R. Ambikapathi, E. H. Lee and D. Olszyk, Environ. Toxicol. Chem., 2008, 27, 1922–1931 CrossRef.
- P. Begum, R. Ikhtiari and B. Fugetsu, Carbon, 2011, 49, 3907–3919 CrossRef CAS.
- M. Kawai-Yamada, Y. Ohori and H. Uchimiya, Plant Cell, 2004, 16, 21–32 CrossRef CAS PubMed.
- X.-M. Tan, C. Lin and B. Fugetsu, Carbon, 2009, 47, 3479–3487 CrossRef CAS.
- P. Begum, R. Ikhtiari, B. Fugetsu, M. Matsuoka, T. Akasaka and F. Watari, Appl. Surf. Sci., 2012, 262, 120–124 CrossRef CAS.
- H. Villagarcia, E. Dervishi, K. de Silva, A. S. Biris and M. V. Khodakovskaya, Small, 2012, 8, 2328–2334 CrossRef CAS PubMed.
- B. D. Soane, B. C. Ball, J. Arvidsson, G. Basch, F. Moreno and J. Roger-Estrade, Soil Tillage Res., 2012, 118, 66–87 CrossRef.
- M. Arias-Estévez, E. López-Periago, E. Martínez-Carballo, J. Simal-Gándara, J.-C. Mejuto and L. García-Río, Agric., Ecosyst. Environ., 2008, 123, 247–260 CrossRef.
- K. Fenner, S. Canonica, L. P. Wackett and M. Elsner, Science, 2013, 341, 752–758 CrossRef CAS PubMed.
- M. A. Beketov, B. J. Kefford, R. B. Schäfer and M. Liess, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 11039–11043 CrossRef CAS PubMed.
- S. Mostafalou and M. Abdollahi, Toxicol. Appl. Pharmacol., 2013, 268, 157–177 CrossRef CAS PubMed.
- H. C. Oliveira, R. Stolf-Moreira, C. B. R. Martinez, R. Grillo, M. B. de Jesus and L. F. Fraceto, PLoS One, 2015, 10, e0132971 Search PubMed.
- S. Kumar, N. Chauhan, M. Gopal, R. Kumar and N. Dilbaghi, Int. J. Biol. Macromol., 2015, 81, 631–637 CrossRef CAS PubMed.
- C. Sun, K. Shu, W. Wang, Z. Ye, T. Liu, Y. Gao, H. Zheng, G. He and Y. Yin, Int. J. Pharm., 2014, 463, 108–114 CrossRef CAS PubMed.
- M. L. Paret, A. J. Palmateer and G. W. Knox, HortScience, 2013, 48, 189–192 CAS.
- M. L. Paret, G. E. Vallad, D. R. Averett, J. B. Jones and S. M. Olson, Phytopathology, 2013, 103, 228–236 CrossRef CAS PubMed.
- S. W. Kim, J. H. Jung, K. Lamsal, Y. S. Kim, J. S. Min and Y. S. Lee, Mycobiology, 2012, 40, 53–58 CrossRef CAS PubMed.
- M. Buteler, S. Sofie, D. Weaver, D. Driscoll, J. Muretta and T. Stadler, Int. J. Pest Manage, 2015, 61, 80–89 CrossRef CAS.
- K. K. Mondal and C. Mani, Ann. Microbiol., 2012, 62, 889–893 CrossRef CAS.
- D. Cai, L. Wang, G. Zhang, X. Zhang and Z. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 9212–9216 CAS.
- S. Sharma, S. Singh, A. K. Ganguli and V. Shanmugam, Carbon, 2017, 115, 781–790 CrossRef CAS.
- A. E. Pereira, R. Grillo, N. F. Mello, A. H. Rosa and L. F. Fraceto, J. Hazard. Mater., 2014, 268, 207–215 CrossRef CAS PubMed.
- M. Kah, S. Beulke, K. Tiede and T. Hofmann, Crit. Rev. Environ. Sci. Technol., 2013, 43, 1823–1867 CrossRef CAS.
- Z. Clemente, R. Grillo, M. Jonsson, N. Santos, L. Feitosa, R. Lima and L. Fraceto, J. Nanosci. Nanotechnol., 2014, 14, 4911–4917 CrossRef CAS PubMed.
- R. Grillo, N. Z. P. dos Santos, C. R. Maruyama, A. H. Rosa, R. de Lima and L. F. Fraceto, J. Hazard. Mater., 2012, 231, 1–9 CrossRef PubMed.
- D. Horn and J. Rieger, Angew. Chem., Int. Ed., 2001, 40, 4330–4361 CrossRef CAS PubMed.
- M. Fathi, M. Mozafari and M. Mohebbi, Trends Food Sci. Technol., 2012, 23, 13–27 CrossRef CAS.
- M. Kah and T. Hofmann, Environ. Int., 2014, 63, 224–235 CrossRef CAS PubMed.
- J. H. Choy, S. J. Choi, J. M. Oh and T. Park, Appl. Clay Sci., 2007, 36, 122–132 CrossRef CAS.
- I. Ravier, E. Haouisee, M. Clément, R. Seux and O. Briand, Pest Manage. Sci., 2005, 61, 728–736 CrossRef CAS PubMed.
- L. C. Jiang, M. Basri, D. Omar, M. B. A. Rahman, A. B. Salleh and R. N. Z. R. A. Rahman, Pestic. Biochem. Physiol., 2012, 102, 19–29 CrossRef CAS.
- C. J. Lim, M. Basri, D. Omar, M. B. Abdul Rahman, A. B. Salleh, R. A. Rahman and R. N. Zaliha, Pest Manage. Sci., 2013, 69, 104–111 CrossRef CAS PubMed.
- E. C. R. da Rodrigues, A. M. Ferreira, J. C. Vilhena, F. B. Almeida, R. A. Cruz, A. C. Florentino, R. N. Souto, J. C. Carvalho and C. P. Fernandes, Rev. Bras. Farmacogn., 2014, 24, 699–705 CrossRef.
- L. Wang, X. Li, G. Zhang, J. Dong and J. Eastoe, J. Colloid Interface Sci., 2007, 314, 230–235 CrossRef CAS PubMed.
- J. L. Duarte, J. R. Amado, A. E. Oliveira, R. A. Cruz, A. M. Ferreira, R. N. Souto, D. Q. Falcão, J. C. Carvalho and C. P. Fernandes, Rev. Bras. Farmacogn., 2015, 25, 189–192 CrossRef CAS.
- B. H. Feng and L. F. Peng, Carbohydr. Polym., 2012, 88, 576–582 CrossRef CAS.
- S. Majumder, N. A. Shakil, J. Kumar, T. Banerjee, P. Sinha, B. B. Singh and P. Garg, J. Environ. Sci. Health, Part B, 2016, 51, 873–880 CrossRef CAS PubMed.
- M. Kah, A.-K. Weniger and T. Hofmann, Environ. Sci. Technol., 2016, 50, 10960–10967 CrossRef CAS PubMed.
- K. Marsh and B. Bugusu, J. Food Sci., 2007, 72, R39–R55 CrossRef CAS PubMed.
- G. L. Robertson, Food packaging: principles and practice, CRC press, 2016 Search PubMed.
- H. M. De Azeredo, Food Res. Int., 2009, 42, 1240–1253 CrossRef CAS.
- R. V. Tauxe, Emerging Infect. Dis., 1997, 3, 425 CrossRef CAS PubMed.
- B. J. McCabe-Sellers and S. E. Beattie, J. Am. Diet. Assoc., 2004, 104, 1708–1717 CrossRef PubMed.
- T. V. Duncan, J. Colloid Interface Sci., 2011, 363, 1–24 CrossRef CAS PubMed.
- C. Silvestre, D. Duraccio and S. Cimmino, Prog. Polym. Sci., 2011, 36, 1766–1782 CrossRef CAS.
- L. D. Mello and L. T. Kubota, Food Chem., 2002, 77, 237–256 CrossRef CAS.
- G. V. Research, Nano-enabled packaging market analysis by technology (active packaging, intelligent & smart packaging, controlled release packaging), by application (food & beverages, pharmaceutical, personal care & cosmetics) and segment forecasts to 2024, Grand View Research, 2016 Search PubMed.
- M. Cushen, J. Kerry, M. Morris, M. Cruz-Romero and E. Cummins, Trends Food Sci. Technol., 2012, 24, 30–46 CrossRef CAS.
- J. Cho, Nano-Ceramic Coated Plastics, NASA Tech Briefs, 2013 Search PubMed.
- B. A. Khan, V. S. Chevali, H. Na, J. Zhu, P. Warner and H. Wang, Composites, Part B, 2016, 100, 10–18 CrossRef CAS.
- S. Farrokhi, H. Ahari and M. Abedini, Int. J. Food Prop., 2017, 20, 1–9 CrossRef.
- J. Castro-Mayorga, M. Fabra and J. Lagaron, Innovative Food Sci. Emerging Technol., 2016, 33, 524–533 CrossRef CAS.
- N. Bumbudsanpharoke, W. Lee and S. Ko, Polym. Compos., 2017 DOI:10.1002/pc.24325.
- M.-J. Khalaj, H. Ahmadi, R. Lesankhosh and G. Khalaj, Trends Food Sci. Technol., 2016, 51, 41–48 CrossRef CAS.
- S. W. Kim and S. H. Cha, J. Appl. Polym. Sci., 2014, 131, 1–8 Search PubMed.
- M. Jorda-Beneyto, N. Ortuño, A. Devis, S. Aucejo, M. Puerto, D. Gutiérrez-Praena, J. Houtman, S. Pichardo, S. Maisanaba and A. Jos, Food Addit. Contam., Part A, 2014, 31, 354–363 CrossRef CAS PubMed.
- H. Staroszczyk, E. Malinowska-Pańczyk, K. Gottfried and I. Kołodziejska, J. Food Process. Preserv., 2017, 41, 1–7 Search PubMed.
- F. Yahiaoui, F. Benhacine, H. Ferfera-Harrar, A. Habi, A. S. Hadj-Hamou and Y. Grohens, Polym. Bull., 2015, 72, 235–254 CrossRef CAS.
- F. Tornuk, M. Hancer, O. Sagdic and H. Yetim, Food Sci. Technol., 2015, 64, 540–546 CAS.
- N. Alipoormazandarani, S. Ghazihoseini and A. M. Nafchi, Carbohydr. Polym., 2015, 134, 745–751 CrossRef CAS PubMed.
- P. Klangmuang and R. Sothornvit, Food Sci. Technol., 2016, 65, 222–227 CAS.
- E. Butnaru, C. N. Cheaburu, O. Yilmaz, G. M. Pricope and C. Vasile, High Perform. Polym., 2016, 28, 1124–1138 CrossRef CAS.
- J. Li, R. Hong, M. Li, H. Li, Y. Zheng and J. Ding, Prog. Org. Coat., 2009, 64, 504–509 CrossRef CAS.
- R. Venkatesan and N. Rajeswari, Polym. Adv. Technol., 2017, 28, 20–27 CrossRef CAS.
- L. Al-Naamani, S. Dobretsov and J. Dutta, Innovative Food Sci. Emerging Technol., 2016, 38, 231–237 CrossRef CAS.
- R. Priyadarshi and Y. S. Negi, J. Polym. Environ., 2016, 1–12 Search PubMed.
- A. Marcous, S. Rasouli and F. Ardestani, Packag. Technol. Sci., 2017, 30, 693–701 CrossRef CAS.
- A. M. Youssef, S. M. El-Sayed, H. S. El-Sayed, H. H. Salama and A. Dufresne, Carbohydr. Polym., 2016, 151, 9–19 CrossRef CAS PubMed.
- Y. A. Arfat, S. Benjakul, T. Prodpran, P. Sumpavapol and P. Songtipya, Food Bioprocess Technol., 2016, 9, 101–112 CrossRef CAS.
- A. M. Díez-Pascual and A. L. Díez-Vicente, ACS Appl. Mater. Interfaces, 2014, 6, 9822–9834 Search PubMed.
- N. A. El-Wakil, E. A. Hassan, R. E. Abou-Zeid and A. Dufresne, Carbohydr. Polym., 2015, 124, 337–346 CrossRef CAS PubMed.
- L. K. Krehula, A. Papić, S. Krehula, V. Gilja, L. Foglar and Z. Hrnjak-Murgić, Polym. Bull., 2017, 74, 1387–1404 CrossRef CAS.
- H. Bodaghi, Y. Mostofi, A. Oromiehie, B. Ghanbarzadeh and Z. G. Hagh, J. Appl. Polym. Sci., 2015, 132, 41764 CrossRef.
- H. M. Moghaddam, M. Khoshtaghaza, A. Salimi and M. Barzegar, Polym.-Plast. Technol. Eng., 2014, 53, 1759–1767 CrossRef CAS.
- Z. Noori Hashemabad, B. Shabanpour, H. Azizi, S. M. Ojagh and A. Alishahi, Polym.-Plast. Technol. Eng., 2017, 1–12 Search PubMed.
- B. Jin, X. Li, X. Zhou, X. Xu, H. Jian, M. Li, K. Guo, J. Guan and S. Yan, RSC Adv., 2017, 7, 16931–16937 RSC.
- S. M. M. Dadfar and G. Kavoosi, Polym. Compos., 2015, 36, 145–152 CrossRef CAS.
- H.-Y. Yu, Z.-Y. Qin, B. Sun, X.-G. Yang and J.-M. Yao, Compos. Sci. Technol., 2014, 94, 96–104 CrossRef CAS.
- S. Paszkiewicz, M. Kwiatkowska, Z. Rosłaniec, A. Szymczyk, M. Jotko and S. Lisiecki, Polym. Compos., 2016, 37, 1949–1959 CrossRef CAS.
- X. Li, C. Qiu, N. Ji, C. Sun, L. Xiong and Q. Sun, Carbohydr. Polym., 2015, 121, 155–162 CrossRef CAS PubMed.
- US EPA, Advancing Sustainable Materials Management: 2014 Fact Sheet, US EPA, Washington, DC, 2016 Search PubMed.
- B. Kuswandi, in Nanoscience in Food and Agriculture 1, Springer, 2016, pp. 151–183 Search PubMed.
- J. C. Buzby, H. F. Wells and J. Hyman, The Estimated Amount, Value, and Calories of Postharvest Food Losses at the Retail and Consumer Levels in the United States, United States Department of Agriculture, 2014 Search PubMed.
- M. H. Wong, J. P. Giraldo, S.-Y. Kwak, V. B. Koman, R. Sinclair, T. T. S. Lew, G. Bisker, P. Liu and M. S. Strano, Nat. Mater., 2017, 16, 264–272 CrossRef CAS PubMed.
- J. P. Giraldo, M. P. Landry, S. M. Faltermeier, T. P. McNicholas, N. M. Iverson, A. A. Boghossian, N. F. Reuel, A. J. Hilmer, F. Sen and J. A. Brew, Nat. Mater., 2014, 13, 400–408 CrossRef CAS PubMed.
- A. N. Misra, M. Misra and R. Singh, Int. J. Pure Appl. Sci. Technol., 2013, 16, 1 CAS.
- Y. Hu, X. Ma, Y. Zhang, Y. Che and J. Zhao, ACS Sensors, 2015, 1, 22–25 CrossRef.
- G. K. Darbha, A. Ray and P. C. Ray, ACS Nano, 2007, 1, 208–214 CrossRef CAS PubMed.
- J. Du, Z. Wang, J. Fan and X. Peng, Sens. Actuators, B, 2015, 212, 481–486 CrossRef CAS.
- G. Chen, W. Chen, Y. Yen, C. Wang, H. Chang and C. Chen, Anal. Chem., 2014, 86, 6843–6849 CrossRef CAS PubMed.
- C. Zhao, G. Zhong, D.-E. Kim, J. Liu and X. Liu, Biomicrofluidics, 2014, 8, 052107 CrossRef PubMed.
- J. M. Slocik, J. S. Zabinski, D. M. Phillips and R. R. Naik, Small, 2008, 4, 548–551 CrossRef CAS PubMed.
- R. E. Luckham and J. D. Brennan, Analyst, 2010, 135, 2028–2035 RSC.
- Y. He, B. Xu, W. Li and H. Yu, J. Agric. Food Chem., 2015, 63, 2930–2934 CrossRef CAS PubMed.
- O. R. Miranda, X. Li, L. Garcia-Gonzalez, Z. J. Zhu, B. Yan, U. H. Bunz and V. M. Rotello, J. Am. Chem. Soc., 2011, 133, 9650–9653 CrossRef CAS PubMed.
- Y. Luan, J. Chen, G. Xie, C. Li, H. Ping, Z. Ma and A. Lu, Microchim. Acta, 2015, 182, 995–1001 CrossRef CAS.
- L. Sheng, J. Ren, Y. Miao, J. Wang and E. Wang, Biosens. Bioelectron., 2011, 26, 3494–3499 CrossRef CAS PubMed.
- Z. Guo, J. Ren, J. Wang and E. Wang, Talanta, 2011, 85, 2517–2521 CrossRef CAS PubMed.
- S. S. Miao, M. S. Wu, L. Y. Ma, X. J. He and H. Yang, Talanta, 2016, 158, 142–151 CrossRef CAS PubMed.
- M. Govindasamy, V. Mani, S.-M. Chen, T.-W. Chen and A. K. Sundramoorthy, Sci. Rep., 2017, 7, 46471 CrossRef CAS PubMed.
- M. Govindasamy, R. Umamaheswari, S.-M. Chen, V. Mani and C. Su, J. Electrochem. Soc., 2017, 164, B403–B408 CrossRef CAS.
- M. Liang, K. Fan, Y. Pan, H. Jiang, F. Wang, D. Yang, D. Lu, J. Feng, J. Zhao and L. Yang, Anal. Chem., 2012, 85, 308–312 CrossRef PubMed.
- Y. F. Huang, Y. F. Wang and X. P. Yan, Environ. Sci. Technol., 2010, 44, 7908–7913 CrossRef CAS PubMed.
- Y. S. Xia and C. Q. Zhu, Talanta, 2008, 75, 215–221 CAS.
- E. Zor, E. Morales-Narváez, A. Zamora-Gálvez, H. Bingol, M. Ersoz and A. Merkoçi, ACS Appl. Mater. Interfaces, 2015, 7, 20272–20279 CAS.
- S. Z. Hossain, C. Ozimok, C. Sicard, S. D. Aguirre, M. M. Ali, Y. Li and J. D. Brennan, Anal. Bioanal. Chem., 2012, 403, 1567–1576 CrossRef CAS PubMed.
- J. Joo, C. Yim, D. Kwon, J. Lee, H. H. Shin, H. J. Cha and S. Jeon, Analyst, 2012, 137, 3609–3612 RSC.
- L. De Stefano, L. Moretti, I. Rendina and L. Rotiroti, Sens. Actuators, B, 2005, 111, 522–525 CrossRef.
- G. Liu, J. Wang, R. Barry, C. Petersen, C. Timchalk, P. L. Gassman and Y. Lin, Chem. – Eur. J., 2008, 14, 9951–9959 CrossRef CAS PubMed.
- Y. Lin, F. Lu and J. Wang, Electroanalysis, 2004, 16, 145–149 CrossRef CAS.
- S. Wu, F. Huang, X. Lan, X. Wang, J. Wang and C. Meng, Sens. Actuators, B, 2013, 177, 724–729 CrossRef CAS.
- A. Vergara, E. Llobet, J. Ramírez, P. Ivanov, L. Fonseca, S. Zampolli, A. Scorzoni, T. Becker, S. Marco and J. Wöllenstein, Sens. Actuators, B, 2007, 127, 143–149 CrossRef CAS.
- C. Singh, S. Srivastava, M. A. Ali, T. K. Gupta, G. Sumana, A. Srivastava, R. Mathur and B. D. Malhotra, Sens. Actuators, B, 2013, 185, 258–264 CrossRef CAS.
- B. A. Wender, R. W. Foley, V. Prado-Lopez, D. Ravikumar, D. A. Eisenberg, T. A. Hottle, J. Sadowski, W. P. Flanagan, A. Fisher, L. Laurin, M. E. Bates, I. Linkov, T. P. Seager, M. P. Fraser and D. H. Guston, Environ. Sci. Technol., 2014, 48, 10531–10538 CrossRef CAS PubMed.
- D. E. Meyer and V. K. Upadhyayula, Clean Technol. Environ. Policy, 2014, 16, 757–772 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |
