Harnessing the power of microwaves for inactivating Pseudomonas aeruginosa with nanohybrids†
Jaime
Plazas-Tuttle‡
 ,
Dipesh
Das
,
Indu V.
Sabaraya
and
Navid B.
Saleh
,
Dipesh
Das
,
Indu V.
Sabaraya
and
Navid B.
Saleh
 *
*
Department of Civil, Architectural and Environmental Engineering, University of Texas at Austin, Austin, TX 78712, USA. E-mail: navid.saleh@utexas.edu; Tel: +(512) 471 9175
First published on 9th November 2017
Abstract
Due to the position of microwave (MW) radiation in the electromagnetic spectrum, it has not yet been successfully utilized to inactivate waterborne microorganisms at a reasonable (energy) cost. Exceptional properties at the nano-scale, namely MW absorption-abilities of carbon nanotubes and excellent spectral conversion-capabilities of lanthanide series metal oxides in concert, hold promise to overcome the energetic barrier of this widely used and affordable technology. This study reports the synthesis of a nano-heterostructure that combines carbon nanotubes' and erbium oxide's properties to generate reactive oxygen species (ROS) and inactivate Pseudomonas aeruginosa. Detailed characterization of the synthesized nanohybrid (NH) material with electron microscopy, X-ray techniques, and thermal gravimetric analysis confirms effective hybridization. At least one log unit of microbial inactivation was achieved via ROS generation with only 20 s of MW irradiation at 110 W (0.0006 kW h energy use), using a conventional MW oven. Inactivation studies with ROS scavenger molecules prove that the generated oxygen species played the dominant role in bacterial inactivation. These breakthrough results hold promise to enable an unintended use (i.e., disinfection) of MW technology, which is diffused deep into the global societal fabric.
Environmental significanceThis article presents the design, synthesis, and environmental application of a novel nano- heterostructure that can be used for bacterial inactivation in water with microwave irradiation. Microwaves are the least energy-intensive radiation that is not capable of being effective for inactivating pathogens, unless they are irradiated for a long-time. The nano-hybrid designed herein can harness the microwave energy and inactivate at least one log of Pseudomonas aeruginosa via reactive oxygen species generation with only 20 s of irradiation at 110 W (0.0006 kW h energy use). The results presented herein can lead to the development of a novel nano-enabled water disinfection technology. |
1 Introduction
The Schumpeterian trilogy of technological change, i.e., invention, innovation, and diffusion, highlights the importance and benefits of societal acceptance of any new technology.1 Once a technology has diffused deep into the societal fabric, the spectrum of its application expands and allows unintended uses, some of which might be transformative. One such example is mobile communication, which was not originally engineered to assist in healthcare, but now is utilized to disseminate medical information and has transformed this sector globally.2,3 Microwave (MW) technology is affordable and similar in social adoptability and thus can be utilized to impact low-income communities across the globe, particularly to gain them access to safe drinking water. Although the position of MW radiation in the electromagnetic spectrum precludes its direct use in inactivating waterborne pathogens at a reasonable cost, finding a way to harness the power of MW radiation in an effective antimicrobial technology could potentially benefit a large global population.Ensuring water safety via disinfection4 has largely relied on the use of chemical oxidants since the early 1900s.5 Commonly used types of such chemicals include chlorine,6 chlorine dioxide,7 and ozone.8 Ammonia added simultaneously or consecutively with chlorine forms another common disinfectant, chloramine, which is less effective, but more persistent compared to chlorine.6 However, chemical disinfectants lead to the production of disinfection by-products (DBPs), which have raised public health concerns since the early 1970s.9 Alternative non-chemical based disinfection technologies became necessary, and ultraviolet (UV) irradiation has been developed as an effective disinfection alternative.10 UV's germicidal effect is a result of the UV action on the nucleic acids of microorganisms and its efficacy depends on light intensity and exposure time.11 Disadvantages of UV technology are the absence of disinfection residual beyond the treatment facility,10,12 its need for a clear optical pathway to enable UV ray penetration,13 and maintenance to prevent fouling of lamps.14 Furthermore, UV technology is not commonly available at every household,15 and rather needs to be custom-made with the purpose of disinfecting water. Microwave-enabled disinfection technology can be a long-lasting, easy to use, and cost-effective alternative. The growth of the global microwave oven market in the near future16 will enable reaping of the unanticipated benefits of pathogen inactivation using this novel technology. Furthermore, such a technology can be used in a community basis where either access to electricity is limited or affordability of such a device is out of range at a household level.
Irradiation-based disinfection technologies are gaining popularity because of advances in equipment reliability and reduction of undesirable disinfection by-products.12,17 The rapid growth of nanotechnology has prompted significant interest in environmental applications, and nano-scale materials are now being incorporated into such irradiation-based disinfection devices to improve reliability, reduce operating costs, and increase their disinfection efficiency.4,17–19 Nanoparticles are used as photocatalysts to enhance and accelerate the inactivation rate of pathogenic microorganisms.18,19 Light irradiated onto photocatalytic materials can effectively generate reactive oxygen species (ROS), one of the key modes of disinfection.4,20 Of particular interest are combinations of materials and irradiation systems that use low-cost visible and/or UV light to achieve high disinfection capacity. However, efficiency of any such technology depends on incident flux and wavelength of the radiation, specific water characteristics, absorption length in water, reactor geometry and hydrodynamics, contact efficiency of species in water and the photocatalysts, and inactivation kinetics.4
The enhancement of low-energy electromagnetic radiation, e.g., visible and near infrared radiation, to produce ROS21 is of particular interest, as a part of the on-going effort to develop new alternative antimicrobial technologies. Such amplification of low energy photons to higher energy22 has been successfully demonstrated using lanthanide series metals (e.g., Er3+ and Tm3+).23,24 Their unique 4fn (n = 0–14) 5d0–1 inner shell configurations are well shielded by the outer filled 5s25p6 sub-shell electrons and thus have an abundance of unique energy levels. When populated, these states can be long lived (up to 0.1 s), making these ideal to serve as electron donors.25 To date, successful utilization of low energy MW radiation for efficient generation of ROS and, thus, inactivation of pathogens has not been demonstrated.
Developed during the Second World War, low-frequency MWs (at least 5 orders of magnitude lower than UV) have disseminated into industry and later into the household consumption market in a short period of time.26 MWs lie between infrared radiation and radio frequencies and correspond to wavelengths of 10−3 to 1 m (300 GHz to 300 MHz frequencies, respectively).27 In this region, the energy of the MW photons (between 1.24 × 10−3 and 1.24 × 10−6 eV) is too weak to break chemical bonds,28 when compared to that of photons emitted by UV lamps with wavelengths ranging between 200 and 280 nm (6.20 to 4.43 eV). However, even this apparently weak MW radiation has proven to be germicidal when used at high intensity and for an extended period of time in sterilizing dry materials.29 MW technology is effectively used to sterilize dentures and dental tools/devices,26 where 10 min of microwaving at 720 W is required for sterilization.30,31 The antimicrobial impact of MW radiation is not well understood, but it is hypothesized to emanate mostly from thermal action32 and also from dielectric rupture.33 However, disinfecting water with MW has not been successful to date, likely due to the extended irradiation period that would be required and associated energy costs.
An alternative irradiation-based antimicrobial technology that can take advantage of an already adopted, affordable, and available device can be greatly beneficial. In this study, the MW absorption-potential of carbon nanotubes has been combined with the spectral conversion-ability of lanthanide series metal oxides and the resultant pathogen inactivation potency is demonstrated. A novel nanohybrid (NH),34,35 multiwalled carbon nanotubes (MWNTs) chemically hybridized with erbium (Er3+) oxide, has been synthesized using a sol–gel method. The material has been characterized to confirm hybridization and determine its physicochemical properties via high-resolution transmission electron microscopy (HRTEM), scanning transmission electron microscopy (STEM), thermal gravimetric analysis (TGA), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). This paper presents breakthrough inactivation results of a common opportunistic environmental organism, P. aeruginosa. The mechanism of inactivation has been enumerated by determining ROS generation and by confirming its role with ROS scavengers, while eliminating other possible mechanisms by accounting for appropriate controls. The results obtained from this study can be considered as the ‘invention’ of a new irradiation-based antimicrobial technology for water that demonstrates promise for utilization of widely available MW technology as a potential disinfection device.
2 Experimental
2.1 Materials
Isopropanol (2-propanol, 99%, USP), nitric acid (70%), and concentrated sulfuric acid were obtained from Fisher Scientific (Houston, TX). MWNTs (>95% carbon purity) with an average diameter of 8–15 nm and length of 10–50 μm were obtained from Cheap Tubes Inc., (Cambridgeport, VT). Amplex® UltraRed reagent (cat. no. A36006) and Amplex® Red/UltraRed stop reagent (cat. no. A33855) were procured from Invitrogen (Carlsbad, CA). Erbium(III) oxide (99.5%, REO) was purchased from Alfa Aesar™ (Ward Hill, MA) while erbium(III) nitrate pentahydrate (99.9% trace metal basis) was procured from Acros Organics™ (Geel, Belgium). Catalase (CAT), superoxide dismutase (SOD), and methanol (MET, 99.9%) were obtained from Sigma (St. Louis, MO). Other reagents were purchased from Fisher Scientific (Houston, TX), unless otherwise noted.2.2 Synthesis of NHs
MWNTs with an average diameter of 8–15 nm and >95% purity (Cheap Tubes Inc., Cambridgeport, VT) were first acid-etched by refluxing in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of concentrated nitric (70%) and sulfuric acid (96.5%) at 80 °C for 3 h. Functionalized MWNTs were thoroughly washed with ultrapure water (Synergy ultrapure water, EMD Millipore, Darmstadt, Germany) and vacuum-filtered using porous polytetrafluoroethylene (PTFE) membrane filters (0.2 μM, EMD Millipore, Darmstadt, Germany). The MWNT cake obtained was washed with distilled water until the pH was neutral, dried in a desiccator, and subsequently hand-ground with mortar and pestle to fine powder to be dispersed in anhydrous isopropanol with an ultrasonic dismembrator (Q700 Qsonica, Newtown, CT). NHs with three C
1 (v/v) mixture of concentrated nitric (70%) and sulfuric acid (96.5%) at 80 °C for 3 h. Functionalized MWNTs were thoroughly washed with ultrapure water (Synergy ultrapure water, EMD Millipore, Darmstadt, Germany) and vacuum-filtered using porous polytetrafluoroethylene (PTFE) membrane filters (0.2 μM, EMD Millipore, Darmstadt, Germany). The MWNT cake obtained was washed with distilled water until the pH was neutral, dried in a desiccator, and subsequently hand-ground with mortar and pestle to fine powder to be dispersed in anhydrous isopropanol with an ultrasonic dismembrator (Q700 Qsonica, Newtown, CT). NHs with three C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er molar ratios, NH-1 (16
Er molar ratios, NH-1 (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), NH-2 (8
1), NH-2 (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and NH-3 (4
1), and NH-3 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), were synthesized (Table S1†) via a sol–gel process.36 For this purpose, the erbium precursor (Er(NO3)3·5H2O) was dissolved in isopropanol (Table S1†), bath sonicated, and added drop-wise at a constant rate (0.435 mL min−1) to the previously dispersed MWNTs under ultra-high purity N2 (NI UHP15A, Airgas) at 80 °C for 3 h. The mixture was then evaporated and the residue was calcined under N2 at 400 °C for 3 h in a tube furnace (Lindberg/Blue M TF55035A-1, Thermo Scientific, Asheville, NC). Finally, the NHs were hand-ground with mortar and pestle and dispersed ultrasonically in ultrapure water before use. All chemicals and materials used were purchased from Fisher Scientific (Houston, TX) unless otherwise noted.
1), were synthesized (Table S1†) via a sol–gel process.36 For this purpose, the erbium precursor (Er(NO3)3·5H2O) was dissolved in isopropanol (Table S1†), bath sonicated, and added drop-wise at a constant rate (0.435 mL min−1) to the previously dispersed MWNTs under ultra-high purity N2 (NI UHP15A, Airgas) at 80 °C for 3 h. The mixture was then evaporated and the residue was calcined under N2 at 400 °C for 3 h in a tube furnace (Lindberg/Blue M TF55035A-1, Thermo Scientific, Asheville, NC). Finally, the NHs were hand-ground with mortar and pestle and dispersed ultrasonically in ultrapure water before use. All chemicals and materials used were purchased from Fisher Scientific (Houston, TX) unless otherwise noted.
2.3 NH characterization
A JEOL 2010F HRTEM (JEOL USA Inc., Pleasanton, CA) at various magnifications and at an accelerating voltage of 200 kV was used to collect MWNT and NH images. The same equipment was used to obtain high annular angle dark field STEM images at high magnification alongside with EDX to obtain elemental mapping of the materials. Crystalline structures of the powdered samples were investigated by performing XRD using a Rigaku R-axis Spider (Rigaku Americas Corporation, The Woodlands, TX). The XRD has a curved imaged plate diffractometer equipped with an image plate detector and Cu-Kα irradiator (0.154 nm wavelength) and a graphite monochromator. Thermal oxidation properties were examined using a TGA with differential scanning calorimetric capabilities (Mettler-Toledo AG, Schwerzenbach, Switzerland). TGA was performed by flowing air from 25 to 800 °C with a heating ramp of 10 °C min−1. XPS (Kratos Axis Ultra DLD, Kratos Analytical Ltd., Manchester, UK) spectra were recorded on dry powders to examine the surface chemistry of the samples.2.4 Antimicrobial potency
Antimicrobiality was assessed by exposing a Gram-negative opportunistic pathogenic strain of P. aeruginosa (PAO1) to the NHs with appropriate controls. P. aeruginosa is ubiquitous in the natural environment, resistant to conventional disinfection, and its biofilm form protects it from chemical disinfectants, UV light, and other environmental stressors;37 these attributes make it a perfect model microorganism that is more resistant than other planktonic bacteria.37 A freezer stock of PAO1 was streaked on a Luria Bertani (LB) agar plate and grown overnight. A single colony from the plate was inoculated in 15 mL LB medium and incubated at 37 °C on a shaker (200 rpm) for 16 h. 100 μL of the culture was added to a fresh LB medium and was incubated at 37 °C for 4–6 h until the culture reached the mid-exponential phase (optical density at 600 nm of 0.25–0.30). The suspension was then centrifuged (5810R, Eppendorf AG, Hamburg, Germany) at 2500×g for 15 min, and the supernatant was removed. The remaining cell residue was re-suspended in 15 mL of a 1X Gibco™ phosphate buffer saline (PBS) solution (Fisher Scientific, Pittsburgh, PA). This procedure of centrifugation and re-suspension in PBS media was repeated twice to remove the remaining LB growth medium. Concentrations of 10 mg L−1 erbium salts, erbium oxide, MWNTs, and NHs samples were prepared in 1X PBS as stocks. Each sample was autoclaved and bath sonicated for 30 min prior to the exposure studies. A 20 μL sample was then added to 180 μL of the bacterial suspension (in PBS) on a microtiter plate to achieve a final bacterial exposure concentration of 1 mg L−1 for each sample. A control was also prepared by adding 20 μL sterile ultrapure DI water to the bacterial suspension to account for the same dilution as that of the other samples. Bacterial suspensions were then subjected to MW irradiation (20 s at 110 W), while an identical set of samples was kept in the dark for the same exposure time. Each sample was tested in triplicate. The samples were then serially diluted using 1X PBS, 10 μL samples were pipetted and grown on LB agar plates, incubated for 12–16 h at 37 °C, and finally colonies were enumerated by direct count.2.5 Antimicrobial mechanism determination
During nanomaterial exposure, bacteria can experience stress from a selected set of stressors, among which dissolved metal ions and ROS are the most common. Bacteria can also be stressed via heat shock and other chemical stressors, e.g., hydrogen peroxide. To identify the dominant underlying mechanism for inactivation of bacteria, the following protocols are established.3 Results and discussion
3.1 Synthesis and characterization of the NHs
NHs with three C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+ molar ratios, i.e., 16
Er3+ molar ratios, i.e., 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NH-1), 8
1 (NH-1), 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NH-2), and 4
1 (NH-2), and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
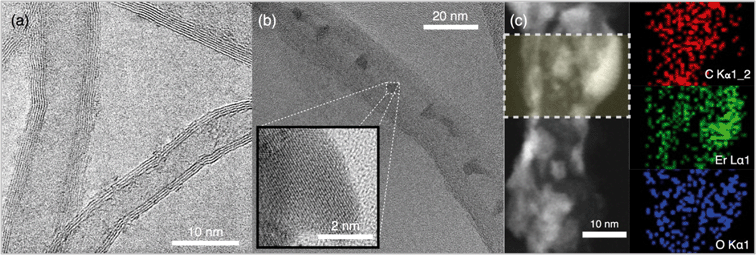
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NH-3), are synthesized (Table S1†) via a sol–gel process. Representative HRTEMs and STEM micrographs show successful hybridization of MWNTs with Er (Fig. 1 and S1†). The HRTEM micrograph displays debundled MWNTs with an average shell thickness of 21.3 ± 2.6 nm (Fig. 1a), where crystalline features are uniformly distributed at the surfaces of the MWNTs indicating hybridization with a metal/metal oxide nanocrystal (Fig. 1b and S1†). The elemental composition of the hybridized MWNTs and the uniformity of the metal oxide nanocrystals are presented via STEM imaging (Fig. 1c). Representative STEM element-specific micrographs show uniform distribution for C, Er, and O, throughout the MWNT backbone. Control over synthesis with loading and distribution uniformity of erbium oxide on MWNTs is demonstrated via STEM images, elemental mapping, and elemental composition (Table S2 and Fig. S2†).
1 (NH-3), are synthesized (Table S1†) via a sol–gel process. Representative HRTEMs and STEM micrographs show successful hybridization of MWNTs with Er (Fig. 1 and S1†). The HRTEM micrograph displays debundled MWNTs with an average shell thickness of 21.3 ± 2.6 nm (Fig. 1a), where crystalline features are uniformly distributed at the surfaces of the MWNTs indicating hybridization with a metal/metal oxide nanocrystal (Fig. 1b and S1†). The elemental composition of the hybridized MWNTs and the uniformity of the metal oxide nanocrystals are presented via STEM imaging (Fig. 1c). Representative STEM element-specific micrographs show uniform distribution for C, Er, and O, throughout the MWNT backbone. Control over synthesis with loading and distribution uniformity of erbium oxide on MWNTs is demonstrated via STEM images, elemental mapping, and elemental composition (Table S2 and Fig. S2†).
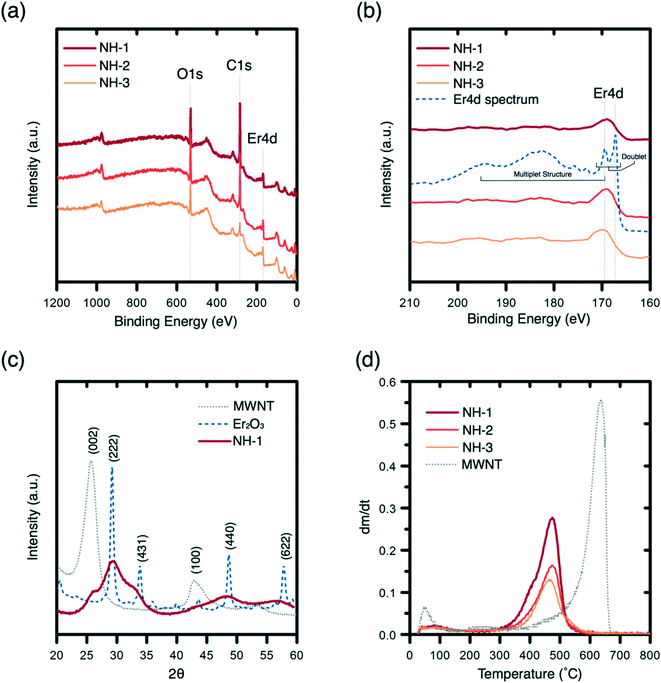
Quantitation of elemental composition for the NHs is presented with XPS analysis (Fig. 2a). XPS spectra for the NHs with varied Er loading reveal the presence of characteristic O 1s, C 1s, and Er 4d peaks (Fig. 2a). The O1s peaks (at 532 eV) are narrow and confirm the presence of different forms of erbium oxide and C–O bonds on the surface of the MWNTs. The C1s (at 284.8 eV) peaks are typical for sp3 hybridized C–C bonds. The region of Er 4d does not exhibit a typical free ion doublet structure at the region between binding energies of 167.5 and 169.5 eV, and the complex multiplet structure to the left of the peak at 169.5 eV is attenuated as shown by the 3 NH signals (Fig. 2b).38 However, the peaks at binding energy 169 eV are typical of the compound Er2O3, which will change the spacing and intensity of the doublet peaks after an annealing process.38 The C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+ atomic ratios obtained (Table S3†) via XPS are 1.29 (NH-1), 0.72 (NH-2), and 0.19 (NH-3), which demonstrate achieving control over the hybridization process.
Er3+ atomic ratios obtained (Table S3†) via XPS are 1.29 (NH-1), 0.72 (NH-2), and 0.19 (NH-3), which demonstrate achieving control over the hybridization process.
The crystallinity of erbium oxides on MWNT surfaces is assessed with XRD spectra (Fig. 2c). The MWNT XRD spectrum (gray) shows a distinctive sharp peak and small broad peaks at 26.3° and 43°, which correspond to (002) and (100) lattice planes, respectively.39 The XRD spectrum of erbium oxide (dash blue) shows the occurrence of broad shoulders at (222), (211), (431), (440), and (622), which are literature reported planes for crystalline Er2O3.40 The lack of peak occurrences at these planes indicates the presence of some amorphous Er2O3 on MWNT surfaces. Since other characterization of the NH showed evidence of crystalline Er2O3 (lattice planes in the electron micrographs in Fig. 1), it can be concluded that the metal oxide on NHs is present both in crystalline and amorphous phases.
To determine whether the erbium oxide nanocrystals crystallized onto MWNT surfaces with no chemical bonding or rather true hybridization has been achieved, peak oxidation temperature of the MWNTs and NHs is determined. TGA results (Fig. 2d) show a significant downward shift of the peak oxidation temperature (from 636 °C to 475 °C) for MWNTs upon hybridization. Such a shift can be attributed to enhanced heat flow onto MWNT surfaces via chemically bonded metallic nanocrystals.41,42 The downward shift in the peak temperature persisted with the increase in the erbium oxide content, which further supports the heat flow analysis. Analyzing the percent mass loss profiles of these materials reveals mass remaining percentages of MWNTs and the NHs, i.e., 6.8% (MWNT), 48.1% (NH-1), 60.7% (NH-2), and 73.2% (NH-3), which concur well with the metal content analysis obtained from EDX (Table S2†). The differences between XPS and TGA/EDX ratios for C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+ suggest that erbium oxides are being crystallized not only on the surfaces but might also be incorporated within the carbon tubules as may be observed in Fig. 1b.
Er3+ suggest that erbium oxides are being crystallized not only on the surfaces but might also be incorporated within the carbon tubules as may be observed in Fig. 1b.
3.2 Antimicrobial potency
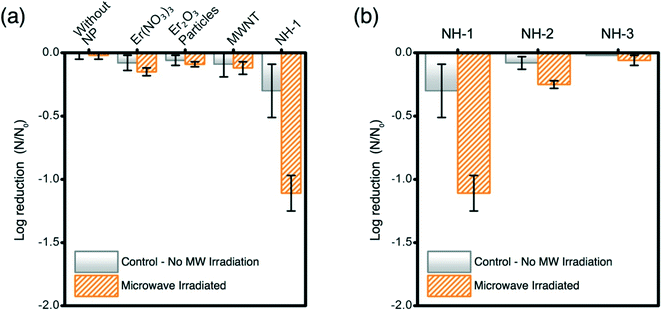
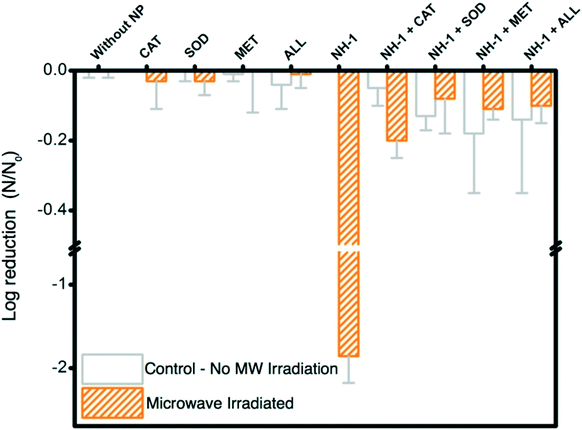
Inactivation of opportunistic pathogen P. aeruginosa with an initial population density of ∼107 CFU mL−1 is successfully achieved with MW irradiation in the presence of NHs (Fig. 3). The control samples (irradiated and non-irradiated Er salt, Er oxide particles, and MWNTs) show no significant impact on bacterial inactivation (Fig. 3a). NH-1 shows at least one log unit reduction of P. aeruginosa when compared to appropriate unirradiated controls and other irradiated materials. Inactivation of P. aeruginosa with other samples is not observed. The increase in Er oxide loading onto MWNTs (irradiated samples) shows a negative correlation with bacterial viability reduction (Fig. 3b).Microwaves' potency for inactivating P. aeruginosa compares well with literature reports; however, such reports are based on photocatalytic disinfection of water as a function of a complex set of variables. Our study presents breakthrough inactivation results, and as such, comparison of these results with other reported data is presented to highlight its potential to compete with other irradiation-based technologies. Literature evidence suggests that strains of P. aeruginosa (AOH1 and NCIMB 10421), when exposed to photocatalytic Ag–TiO2 films and irradiated with UV for at least 1–6 h, can result in one log bacterial reduction (energy expenditure: 2.24 mW cm−2).43 Similarly single log inactivation of P. aeruginosa (NCTC 10662) was also achieved by photocatalytic TiO2 thin film treatment, when irradiated with UV (3 mW cm−2) for 35 min.44 Comparable inactivation efficiency of P. aeruginosa (ATCC 9027) is observed for solar irradiated TiO2 when irradiated for 1 h (energy expenditure: 1 kW h).45Escherichia coli (OH157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H7), a more susceptible bacterial species to irradiative inactivation (compared to P. aeruginosa), underwent single log inactivation with C70-modified TiO2 NHs under 10 min irradiation of visible light (energy expenditure: 0.05 kW h). The results presented herein demonstrate superior inactivation performance of the novel NHs prepared in this study, where an opportunistic pathogenic strain is irradiated with the lowest intensity electromagnetic radiation, MWs. In this study, a significant reduction in the exposure time (20 s) and expended energy (0.0006 kW h) compared to literature reported UV and visible radiation excited nanomaterial cases further proves the efficacy and transformative nature of this nano-enabled antimicrobial technology.
H7), a more susceptible bacterial species to irradiative inactivation (compared to P. aeruginosa), underwent single log inactivation with C70-modified TiO2 NHs under 10 min irradiation of visible light (energy expenditure: 0.05 kW h). The results presented herein demonstrate superior inactivation performance of the novel NHs prepared in this study, where an opportunistic pathogenic strain is irradiated with the lowest intensity electromagnetic radiation, MWs. In this study, a significant reduction in the exposure time (20 s) and expended energy (0.0006 kW h) compared to literature reported UV and visible radiation excited nanomaterial cases further proves the efficacy and transformative nature of this nano-enabled antimicrobial technology.
3.3 Proposed antimicrobial mechanisms
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
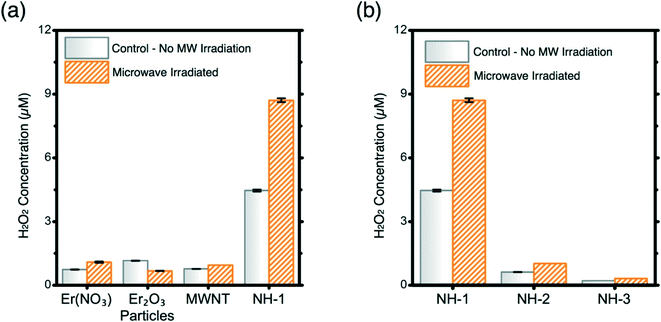
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) is the most effective of the 3 NHs synthesized in producing H2O2 (Fig. 4b). The increase in Er loading on MWNTs negatively correlates with the ROS production ability, as presented in Fig. 3b. NH-2 and NH-3 do not produce significant amounts of H2O2 compared to NH-1. Balance between the MW absorption ability of the MWNTs and the electron donation capacity of the metal oxides is necessary to achieve enhanced antimicrobiality.
1 molar ratio) is the most effective of the 3 NHs synthesized in producing H2O2 (Fig. 4b). The increase in Er loading on MWNTs negatively correlates with the ROS production ability, as presented in Fig. 3b. NH-2 and NH-3 do not produce significant amounts of H2O2 compared to NH-1. Balance between the MW absorption ability of the MWNTs and the electron donation capacity of the metal oxides is necessary to achieve enhanced antimicrobiality.
3.4 Possible ROS-generation mechanism
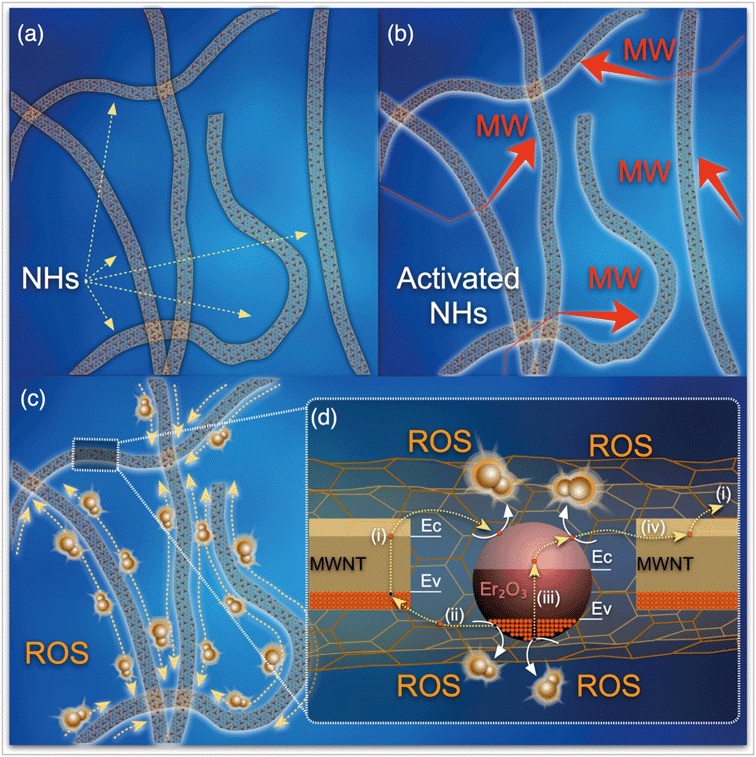
The synergistic effects of MWNT and Er2O3 to favorably generate ROS may be explained with published literature on photocatalytic nanomaterials, e.g., carbon nanotubes–TiO2 nanohybrids.54–61 The overall phenomenon is likely a two-step process of electron promotion and subsequent electron transport to the neighboring MWNT or Er2O3 surfaces with the following possible scenarios. i) The MW absorption ability of MWNTs likely allows the weak and otherwise dissipated MW energy to be localized on the tubular carbon surfaces.62 MWNTs, acting as sensitizers, absorb this energy and as a result electron–hole pairs are generated on the MWNTs (eqn (1) and Fig. 6c). An energized electron from MWNT can get transported to chemically bonded Er2O3’s conduction band allowing the formation of superoxide radicals (eqn (2)). ii) Subsequently, the positively charged MWNTs can remove an electron63 from the valence band of Er2O3, leaving a hole on the metal oxide crystal, which can then react with water to form hydroxyl radicals (eqn (3)). iii) Alternatively, the localized energy may reach sufficient intensity to excite electrons from the Er2O3 valence band to a higher energy state, while leaving a hole behind. The promoted electron on Er2O3 crystals can either react with dissolved oxygen in the surrounding water envelope to form ROS or iv) can get transported to MWNT (due to its exceptional electron transport ability63) and generate ROS via the pathway described earlier. The potential pathways of electron–hole pair formation and ROS generation are illustrated in Fig. 6d and captured in the following set of reactions. It is to be noted that these ROS species undergo further reactions to form hydrogen peroxide (ESI† page S7), which has been measured in this study.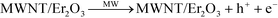 | (1) |
| O2 + e− → O2˙− | (2) |
| H2O + h+ → OH˙ + H+ | (3) |
It is also reported that modification of the electromagnetic properties of MWNTs via hybridization with Er oxide results in improved MW absorbing abilities64 and carbon–oxygen–erbium bonds eventually enable expansion of the electromagnetic absorption range.56 Such phenomena can also lead to improvement in catalytic activity of the NHs.
We acknowledge that generated ROS are temporal in nature and undergo a series of consecutive reactions where these acquire different chemical forms (details in section S1†). H2O2 forms as a reaction product and appears in a later period in the reaction sequence (section S1†). Production of H2O2 in this study is thus likely a result of electron donation from the NHs when irradiated with MW and production of molecular superoxide radicals. It is to be noted that the formation of other ROS is yet to be determined, which will further elucidate the kinetics of oxygen species formation and their subsequent effects in bacterial inactivation. Electron spin resonance spectroscopy with appropriate spin traps can be utilized to determine all ROS generated in this disinfection process.
4 Conclusions
This is the first study that has developed a nano-scale heterostructure, effective in harnessing and utilizing MW radiation for ROS production and microbial inactivation. Synergistic abilities of MWNTs' MW absorption-ability with lanthanide series oxides' spectral conversion-capacity has allowed successful ROS generation. Effective antimicrobiality with ROS utilizing one of the lowest energy radiation (MW) at an exceptionally low energy cost (0.0006 kW h) is potentially transformative. This simple yet elegant technological breakthrough will allow achieving a beneficial unintended use (i.e., bacterial inactivation) from this widely-distributed MW technology. The nascent benefits of MW, i.e., its ability to operate in the absence of clear optical pathways (e.g., in turbid waters), its diffused presence deep into the societal fabric, and its potentially low economic and energetic footprints will allow likely future implementation as an effective point-of-use water treatment solution. The authors acknowledge challenges that this technology will need to overcome to be the panacea and serve as a platform for disinfection processes in the future. Factors such as costs of the technology compared to proven existing disinfection processes, treatable volume of water, higher log-removal of P. aeruginosa and a wide range of waterborne pathogens, material lifespan, and optimal operational parameters are yet to be determined. The mode of application of the material to achieve an effective operational and maintenance level and systematic evaluation of nano environmental health and safety issues are also to be determined. Once the material design and parameters of irradiation are optimized and this technology is further developed as an affordable and effective point-of-use system, it can potentially be transformative to impact a global population by gaining them access to safe drinking water.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is partially supported by an NSF award, bearing award #1602273, and an U.S. EPA award, bearing award #83560201. The authors also thank the Office of the Vice President for Research at UT Austin for partially supporting this work via the 2017's creative grants program within the university. The authors thank Dr. Karalee Jarvis and Dr. Hugo Celio of Texas Materials Institute for their assistance with the HRTEM and STEM imaging and XPS and TGA analyses. The authors also thank Dr. Mary Jo Kirisits for her helpful guidance and members of her research group, Bryant Chambers and Sarah Keithley, for sharing their expertise on P. aeruginosa. We thank the Universidad de los Andes and Fulbright-COLCIENCIAS-DNP for their support to Jaime Plazas-Tuttle.References
- P. Stoneman and P. Diederen, Econ. J., 1994, 104, 918 CrossRef.
- R. S. H. Istepanian, E. Jovanov and Y. T. Zhang, IEEE Trans. Inf. Technol. Biomed., 2004, 8, 405–414 CrossRef PubMed.
- R. T. Lester, P. Ritvo, E. J. Mills, A. Kariri, S. Karanja, M. H. Chung, W. Jack, J. Habyarimana, M. Sadatsafavi, M. Najafzadeh, C. A. Marra, B. Estambale, E. Ngugi, T. B. Ball, L. Thabane, L. J. Gelmon, J. Kimani, M. Ackers and F. A. Plummer, Lancet, 2010, 376, 1838–1845 CrossRef.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- S. D. Richardson, A. D. Thruston Jr, T. V. Caughran, P. H. Chen, T. W. Collette, K. M. Schenck, B. W. Lykins Jr, C. Rav-Acha and V. Glezer, in Environmental Challenges, Springer, Netherlands, Dordrecht, 2000, pp. 95–102 Search PubMed.
- M. Deborde and G. U. von, Water Res., 2008, 42, 13–51 CrossRef CAS PubMed.
- E. M. Aieta and J. D. Berg, J. - Am. Water Works Assoc., 1986, 78, 62–72 CAS.
- J. Hoigne and H. Bader, Water Res., 1976, 10, 377–386 CrossRef CAS.
- S. D. Richardson, TrAC, Trends Anal. Chem., 2003, 22, 666–684 CrossRef CAS.
- R. L. Wolfe, Environ. Sci. Technol., 1990, 24, 768–773 CrossRef CAS.
- R. S. Tobin, D. K. Smith, A. Horton and V. C. Armstrong, J. - Am. Water Works Assoc., 1983, 75, 481–484 Search PubMed.
- H. Zhou and D. W. Smith, Can. J. Civ. Eng., 2001, 28, 49–66 CrossRef.
- J. Christensen and K. G. Linden, J. - Am. Water Works Assoc., 2003, 95, 179–189 CAS.
- D. I. Massé, L. Masse, E. Topp, G. Séguin, L. M. Ortega, A. Scott and É. Pariseau, Water Qual. Res. J. Can., 2011, 46, 2–11 CrossRef.
- M. Parrotta, J. - Am. Water Works Assoc., 1998, 90, 71–81 CAS.
- P. Bisht, Microwave Oven Market by Type (Convection, Grill, Solo), Application (Household, Commercial) and Structure (Built-in, Counter Top) - Global Opportunity Analysis and Industry Forecast, 2014 - 2020, 2015 Search PubMed.
- G. Pyrgiotakis, J. McDevitt, A. Bordini, E. Diaz, R. Molina, C. Watson, G. Deloid, S. Lenard, N. Fix, Y. Mizuyama, T. Yamauchi, J. Brain and P. Demokritou, Environ. Sci.: Nano, 2014, 1, 15–26 RSC.
- W. Wang, G. Li, D. Xia, T. An, H. Zhao and P. K. Wong, Environ. Sci.: Nano, 2017, 4, 782–799 RSC.
- H.-H. Kim, M. S. Kim, H.-E. Kim, H.-J. Lee, M.-H. Jang, J. Choi, Y. Hwang and C. Lee, Environ. Sci.: Nano, 2017, 4, 396–405 RSC.
- S. E. Lehman, A. S. Morris, P. S. Mueller, A. K. Salem, V. H. Grassian and S. C. Larsen, Environ. Sci.: Nano, 2016, 3, 56–66 RSC.
- J.-H. Kim and J.-H. Kim, J. Am. Chem. Soc., 2012, 134, 17478–17481 CrossRef CAS PubMed.
- E. L. Cates, A. P. Wilkinson and J.-H. Kim, J. Phys. Chem. C, 2012, 116, 12772–12778 CAS.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- B. Zhou, B. Shi, D. Jin and X. Liu, Nat. Nanotechnol., 2015, 10, 924–936 CrossRef CAS PubMed.
- J. Chen and J. X. Zhao, Sensors, 2012, 12, 2414–2435 CrossRef CAS PubMed.
- C. Vergani, A. C. Pavarina, D. G. Ribeiro, L. N. Dovigo and P. V. Sanita, Microwave heating, ed. U. Chandra, Microwave Assisted Disinfection Method in Dentistry, 2011, pp. 63–89 Search PubMed.
- D. M. P. Mingos and D. R. Baghurst, Chem. Soc. Rev., 1991, 20, 1–47 RSC.
- M. Grandbois, M. Beyer, M. Rief, H. Clausen-Schaumann and H. E. Gaub, Science, 1999, 283, 1727–1730 CrossRef CAS PubMed.
- M. D. Keller, W. K. Bellows and R. Guillard, J. Exp. Mar. Biol. Ecol., 1988, 117, 279–283 CrossRef.
- K. H. Neppelenbroek, A. C. Pavarina, D. M. Palomari Spolidorio, E. M. Sgavioli Massucato, L. C. Spolidorio and C. E. Vergani, J. Oral Rehabil., 2008, 35, 836–846 CrossRef CAS PubMed.
- L. N. Dovigo, A. C. Pavarina, D. G. Ribeiro, J. A. de Oliveira, C. E. Vergani and A. L. Machado, J. Prosthodontics, 2009, 18, 611–617 CrossRef PubMed.
- C. B. Yeo, I. A. Watson, D. E. Stewart-Tull and V. H. Koh, J. Appl. Microbiol., 1999, 87, 396–401 CrossRef CAS PubMed.
- U. Zimmermann, G. Pilwat and F. Riemann, Biophys. J., 1974, 14, 881–899 CrossRef CAS PubMed.
- N. B. Saleh, N. Aich, J. Plazas-Tuttle, J. R. Lead and G. V. Lowry, Environ. Sci.: Nano, 2015, 2, 11–18 RSC.
- N. B. Saleh, A. Afrooz, J. Bisesi Jr, N. Aich, J. Plazas-Tuttle and T. Sabo-Attwood, Nanomaterials, 2014, 4, 372–407 CrossRef PubMed.
- D. Das, J. Plazas-Tuttle, I. V. Sabaraya, S. S. Jain, T. Sabo-Attwood and N. B. Saleh, Environ. Sci.: Nano, 2017, 4, 60–68 RSC.
- Ö. Kekeç, B. Gökalsın, İ. Karaltı, F. E. Kayhan and N. C. Sesal, Curr. Microbiol., 2016, 73, 228–235 CrossRef PubMed.
- N. Guerfi, O. Bourbia and S. Achour, Mater. Sci. Forum, 2005, 480–481, 193–196 CrossRef CAS.
- W. A. Rigdon and X. Huang, J. Power Sources, 2014, 272, 845–859 CrossRef CAS.
- M. Miritello, R. Lo Savio, A. M. Piro, G. Franzò, F. Priolo, F. Iacona and C. Bongiorno, J. Appl. Phys., 2006, 100, 013502 CrossRef.
- S. Aksel and D. Eder, J. Mater. Chem., 2010, 20(41), 9149–9154 RSC.
- E. Mosquera, D. E. Diaz-Droguett, N. Carvajal, M. Roble, M. Morel and R. Espinoza, Diamond Relat. Mater., 2014, 43, 66–71 CrossRef CAS.
- H. A. Foster, D. W. Sheel, P. Sheel, P. Evans, S. Varghese, N. Rutschke and H. M. Yates, J. Photochem. Photobiol., A, 2010, 216, 283–289 CrossRef CAS.
- P. S. M. Dunlop, C. P. Sheeran, J. A. Byrne, M. A. S. McMahon, M. A. Boyle and K. G. McGuigan, J. Photochem. Photobiol., A, 2010, 216, 303–310 CrossRef CAS.
- J. Lonnen, S. Kilvington, S. C. Kehoe, F. Al-Touati and K. G. McGuigan, Water Res., 2005, 39, 877–883 CrossRef CAS PubMed.
- B. Chambers, A. R. M. N. Afrooz, S. Bae, N. Aich, L. Katz, N. B. Saleh and M. J. Kirisits, Environ. Sci. Technol., 2014, 48, 761–769 CrossRef CAS PubMed.
- Z. Chen, H. Chen, H. Hu, M. Yu, F. Li, Q. Zhang, Z. Zhou, T. Yi and C. Huang, J. Am. Chem. Soc., 2008, 130, 3023–3029 CrossRef CAS PubMed.
- A. O'Toole, E. B. Ricker and E. Nuxoll, Biofouling, 2015, 31, 665–675 CrossRef PubMed.
- T. Kuchma, J. Microwave Power, 1998, 33, 77–87 CAS.
- W. He, H. Jia, J. Cai, X. Han, Z. Zheng, W. G. Wamer and J.-J. Yin, J. Phys. Chem. C, 2016, 120, 3187–3195 CAS.
- P. Sun, C. Tyree and C.-H. Huang, Environ. Sci. Technol., 2016, 50, 4448–4458 CrossRef CAS PubMed.
- Q. Chang, H. He and Z. Ma, J. Inorg. Biochem., 2008, 102, 1736–1742 CrossRef CAS PubMed.
- C. O. Dimkpa, A. Calder, P. Gajjar, S. Merugu, W. Huang, D. W. Britt, J. E. McLean, W. P. Johnson and A. J. Anderson, J. Hazard. Mater., 2011, 188, 428–435 CrossRef CAS PubMed.
- M. R. Hoffmann, S. T. Martin, W. Y. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- W. Wang, P. Serp, P. Kalck and J. L. Faria, J. Mol. Catal. A: Chem., 2005, 235, 194–199 CrossRef CAS.
- K. Woan, G. Pyrgiotakis and W. Sigmund, Adv. Mater., 2009, 21(21), 2233–2239 CrossRef CAS.
- S. Banerjee and S. S. Wong, Nano Lett., 2002, 2, 195–200 CrossRef CAS.
- S. Sun, L. Gao and Y. Liu, Appl. Phys. Lett., 2010, 96, 083113 CrossRef.
- P. V. Kamat, Semiconductor Nanoclusters- Physical, Chemical, and Catalytic Aspects, 1997, vol. 103, pp. 237–259 Search PubMed.
- P. V. Kamat, M. Flumiani and A. Dawson, Colloids Surf., A, 2002, 202, 269–279 CrossRef CAS.
- C. Lettmann, K. Hildenbrand, H. Kisch, W. Macyk and W. F. Maier, Appl. Catal., B, 2001, 32, 215–227 CrossRef CAS.
- E. Vázquez and M. Prato, ACS Nano, 2009, 3, 3819–3824 CrossRef PubMed.
- A. Peigney, C. Laurent, E. Flahaut, R. R. Bacsa and A. Rousset, Carbon, 2001, 39, 507–514 CrossRef CAS.
- L. Zhang, H. Zhu, Y. Song, Y. Zhang and Y. Huang, Mater. Sci. Eng., B, 2008, 153, 78–82 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7en00702g |
| ‡ Current affiliation: Department of Civil and Environmental Engineering, Universidad de los Andes, Carrera 1 Este No. 19 A-40, Edificio ML, Piso 6, Bogotá, Colombia. |
| This journal is © The Royal Society of Chemistry 2018 |