Open Access Article
Open Access ArticlePer- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: implications for environmental emissions and human exposure†
Lara
Schultes
 *a,
Robin
Vestergren
*b,
Kristina
Volkova
c,
Emelie
Westberg
*a,
Robin
Vestergren
*b,
Kristina
Volkova
c,
Emelie
Westberg
 b,
Therese
Jacobson
c and
Jonathan P.
Benskin
b,
Therese
Jacobson
c and
Jonathan P.
Benskin
 *a
*a
aDepartment of Environmental Science and Analytical Chemistry (ACES), Stockholm University, Stockholm, Sweden. E-mail: Lara.Schultes@gmail.com; Jon.Benskin@aces.su.se
bSwedish Environmental Research Institute (IVL), Stockholm, Sweden. E-mail: Robin.Vestergren@ivl.se
cSwedish Society for Nature Conservation (SSNC), Stockholm, Sweden
First published on 1st November 2018
Abstract
Per- and polyfluoroalkyl substances (PFASs) are a diverse class of >4700 chemicals used in commercial products and industrial processes. Concerns surrounding PFASs are principally due to their widespread occurrence in humans and the environment and links to adverse health effects. One of the lesser known uses for PFASs is in cosmetic products (CPs) which come into contact with the skin (e.g. hair products, powders, sunblocks, etc.). In the present work, thirty-one CPs from five product categories (cream, foundation, pencil, powder and shaving foam) were analyzed for 39 PFASs by liquid chromatography-tandem mass spectrometry, as well as extractable organic fluorine (EOF) and total fluorine (TF) by combustion ion chromatography (CIC). This multi-platform approach enabled determination of the fraction of fluorine accounted for by known PFASs (i.e. fluorine mass balance). Foundations and powders contained 25 different PFASs with the most frequently detected being perfluorinated carboxylic acids (perfluoroheptanoic acid and perfluorohexanoic acid) and polyfluoroalkyl phosphate esters (PAPs). Σ14PAP concentrations up to 470 μg g−1 were measured in products listing mixtures of PAPs as an ingredient. For all samples, Σ39PFAS concentrations only explained a small fraction of the EOF and TF, pointing to the presence of unknown organic and/or inorganic fluorinated substances, including polymers. While creams, pencil and shaving foams did not contain measurable concentrations of any of the 39 PFASs targeted here, CIC revealed high to moderate TF content. Overall, these data highlight the need for further investigations into the occurrence of PFASs in CPs and their importance with regards to human and environmental exposure.
Environmental significanceA number of regulatory restrictions and substitution measures have been implemented over the last decade with the aim of reducing environmental emissions and human exposure to per- and polyfluoroalkyl substances (PFASs). However, the large number and structural diversity of PFASs make it difficult to comprehensively assess environmental emissions and human exposure to this class of contaminants. Using a multi-platform approach to facilitate fluorine mass balance calculations, this study demonstrates that Swedish cosmetic products contain large quantities of both known and as-of-yet unidentified PFASs. The results indicate the need for improved understanding of dermal exposure and environmental emissions arising from PFASs in cosmetic products and the importance of fluorine mass balance determination as part of consumer product testing. |
Introduction
Per- and polyfluoroalkyl substances (PFASs) comprise a large and diverse group of synthetic chemicals which have been produced since the 1940s. Due to the strong electronegativity and small atomic size of fluorine, the perfluoroalkyl moiety (–CnF2n+1) imparts unique properties to molecules including high surface activity, chemical and thermal stability and water- and oil-repellency.1 These properties make PFASs useful as low-molecular weight surfactants or polymeric materials in an array of industrial applications and consumer products.2 However; following the detection of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in human blood and wildlife samples from around the globe in 2001 there has been an increasing focus on the adverse environmental properties of PFASs.3,4 Since the early 2000s it has been well-established that long-chain perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs) fulfill regulatory criteria for persistent, bioaccumulative and toxic substances.5 A growing number of toxicological and epidemiological studies also suggest that elevated exposure to PFASs is related to adverse health effects in human populations.6–8Concerns regarding long-term adverse effects of PFASs have led to numerous regulatory actions and industry substitution initiatives for reducing human and wildlife exposure to PFASs.9 A general strategy among fluorochemical producers in the European Union (EU), North America and Japan has involved replacement of long-chain PFCAs, PFSAs and their precursors with shorter-chain homologues or various per- or polyfluoroether compounds10,11 and reduction of point source emissions related to PFAS manufacture.12,13 The industrial transition away from long-chain PFCAs and PFSAs was followed by regulatory restrictions on the quantity of these substances used in consumer products to ensure compliance by the fluorochemical industry. For example, with few exceptions, it is prohibited by EU regulation to manufacture or import products containing more than 10 mg kg−1 of PFOS and its salts.14 A similar restriction of 25 ppb of PFOA and its salts (or 1000 ppb ΣPFOA-related compounds) will be implemented in the EU in 2020.15 These regulations, however, only cover a small number of the PFASs in circulation on the global market. A recent report by the Organization for Economic and Co-operation and Development (OECD) identified 4730 PFAS related CAS numbers, of which 20% are mixtures.16 The database excludes substances with missing CAS numbers or substances under confidential business information, suggesting that the actual number of PFASs might be even larger. For many of the non-regulated substances there is little information about their structure, use, exposure, toxicity, fate and associated risk(s), rendering the process of regulating these chemicals time-consuming.
One of the lesser known uses for PFASs is in cosmetics products (CPs) which come into contact with the skin (e.g. hair products, powders, sunblocks, etc.). Examples of fluorinated ingredients in CPs include: per/polyfluorinated acrylate polymers, naphthalenes, alkanes/alkenes, alcohols, siloxanes, silanes, sulfonamides, ethers, esters, phosphate esters (PAPs), acrylates and acids.17 According to the European Commission's database on cosmetic ingredients (CosIng), these substances are used in CPs as emulsifiers, antistatics, stabilizers, surfactants, film formers, viscosity regulators and solvents.18 Regulation of PFASs included in CPs on the European market falls under the Cosmetics Regulation (Regulation (EC) no. 1223/2009) which stipulates that CP manufacturers must ensure that the contents of their products are safe for human health.19 However, this regulation does not contain requirements on the use of substances that may impact the environment (i.e. which display persistent and bioaccumulative properties). Instead, the cosmetics regulation stipulates that environmental risks should be addressed by REACH (Registration, Evaluation, Authorization and restriction of Chemicals). REACH specifies that polymers and low molecular weight substances imported or manufactured in quantities of <1 tonne per year do not require hazard and risk assessments. Similarly, in the US, the Federal Food, Drug, and Cosmetic Act does not require cosmetic ingredients (with the exception of some color additives) to be approved by the Food and Drug Administration prior to entering the market.20 While CPs are expected to be safe for consumers under their normal conditions of use, assessing the safety of their ingredients ultimately falls on the companies and individuals responsible for marketing them. Thus, PFASs used in CPs may fly under the radar of both EU and North American Cosmetics regulation and REACH, either because there are no data on their risk(s) to human health or because they are used in relatively small quantities.17
Few data are available on the occurrence of PFASs in CPs. While a recent survey of ingredients confirmed the presence of 59 different PFAS-containing CPs on the Swedish market, their concentrations remain unknown.21 In fact, to our knowledge the only study which has quantified PFASs in CPs was that of Fujii et al. (2013) in which >87% of products sampled from Japan, Korea, France and the United States contained PFCAs.22 However; that study only focused on a small subset of known PFASs, and not substances listed on the ingredients lists, as for example PAPs. Clearly, one of the major challenges associated with characterizing CPs (and commercial products in general) involves how to handle the large number and diversity of PFASs. While liquid chromatography coupled to tandem mass-spectrometry (LC-MS/MS) is the method of choice for ionic PFASs, volatile neutral PFASs and polymeric PFASs typically require analysis by gas chromatography (GC)-MS and matrix-assisted laser desorption ionization time-of-flight-MS, respectively.23,24 Despite the availability of these analytical platforms, many PFASs lack authentic standards, making them unquantifiable using the aforementioned approaches. In order to circumvent the problems of multiple MS-based approaches and standard availability, combustion ion chromatography (CIC)25–36 and particle induced gamma ray emission (PIGE)28,37,38 have recently been introduced for indirect quantification of total and extractable organic fluorine (TF and EOF, respectively) in samples. As these approaches are fluorine-specific, a standard of any fluorinated substance may be used for quantification. When paired with targeted PFAS analysis using e.g. LC-MS/MS, fluorine mass balance calculations can be used to assess the fraction of TF and EOF explained by known PFASs, but also the fraction of as-of-yet unidentified PFASs. Early applications of CIC and PIGE in firefighting foams, biota and textiles have revealed that only a small fraction of the organic fluorine content can be accounted for by known PFASs.31,33,37
The overall aim of this study was to quantify a diverse range of PFASs in CPs on the Swedish market and assess the fraction of TF and EOF accounted for by these substances. To achieve this goal we used a combination of targeted LC-MS/MS analysis and fluorine-specific CIC measurements, combined with fluorine mass balance calculations. To our knowledge this is the first fluorine mass balance study on CPs. The results are discussed with respect to their implications for environmental emissions and human exposure.
Methods and materials
Sample selection
Thirty-one CPs from five product categories were analyzed in the present work. Representative samples of PFAS-containing CPs were selected from a database of ingredient lists, compiled by the Swedish Society for Nature Conservation (SSNC). Based on the survey by KEMI in 2015, the most frequently reported PFASs reported in CPs were used to identify specific products containing these substances on their ingredient list.17 During 2016 and 2017, 24 CPs listing 9 different PFASs as active ingredients were purchased from the Swedish market. In addition, 7 products which did not list PFASs among their ingredients were purchased from the same stores as control samples. The products included moisturizing creams (abbreviated CRE), foundations (FOUN), powders and eye shadows (POW), eye pencil (PEN) and shaving foams (SHAV). A tabular overview of the samples, brand names and fluorinated ingredients (according to international nomenclature of cosmetic ingredients (INCI)) as listed on the packaging is given in Table 1.| Sample IDa | Brand | Product name | Fluorinated ingredient |
|---|---|---|---|
| a CRE = cream; FOUN = foundation; PEN = pencil; POW = powder; SHAV = shaving foam. b No fluorinated ingredient listed. | |||
| CRE01 | L'Oréal | Skinperfection, Correcting Day Moisturiser | PTFE |
| CRE02 | Biotherm | Aquasource, rich cream, dry skin | Polyperfluoromethylisopropyl ether |
| CRE03 | Biotherm | Homme, Aquapower, Oligo-thermal comfort care | Polyperfluoromethylisopropyl ether |
| CRE04 | Biotherm | Skin-best, Cream SPF 15, Normal/Combination Skin | PTFE |
| CRE05 | Garnier | The Miracle Cream, SPF 20, all skin types | PTFE |
| CRE06 | Lumene | Beauty lift, illuminating V-shaping serum | Trifluoroacetyl tripeptide-2 |
| CRE07b | Biotherm | Aquasource nutrition, rich balm, very dry skin | — |
| FOUN01 | The Body Shop | Shade adjusting drops darkening | Ammonium C6-16 perfluoroalkylethyl phosphate |
| FOUN02 | Lumene | Nude perfection fluid foundation, 2 Soft Honey, normal to oily skin | C9-15 fluoroalcohol phosphate |
| FOUN03 | The Body Shop | Fresh Nude Foundation SPF 15, 020 Bali Vanilla | Ammonium C6-16 perfluoroalkylethyl phosphate |
| FOUN04 | IsaDora | Hydralight, water-based matte make-up, 57 fair beige | Polyperfluoroethoxymethoxy difluoroethyl PEG phosphate |
| FOUN05 | Lumene | BLUR Foundation longwear, 6 Golden light, all skin types, SPF 15 | Perfluorooctyl triethoxysilane |
| FOUN06 | Sensai | Fluid finish lasting velvet, SPF 15 | Trifluoropropyl demethiconol |
| FOUN07b | Lumene | Invisible illumination, instant glow beauty serum, all skin types | — |
| FOUN08b | IsaDora | Wake up make-up, SPF 20 | — |
| FOUN09b | The Body Shop | Moisture foundation SPF 15 | — |
| PEN01 | H&M | Color Essence Eye Pencil (celestial) | Perfluorononyl dimethicone |
| POW01 | H&M | Eylure Brow Palette, brow trio | PTFE |
| POW02 | IsaDora | Eye color bar | PTFE |
| POW03 | IsaDora | Anti-Shine Mattiflying Powder | Polyperfluoroethoxymethoxy difluoroethyl PEG phosphate |
| POW04 | H&M | Face Palette (4 colors) | Polytefum (PTFE), polytef (PTFE) |
| POW05b | IsaDora | Bronzing powder | — |
| POW06 | Lumene | Luminous matt | Perfluorooctyl triethoxysilane |
| POW07b | H&M | Blusher highlighter palette | — |
| POW08 | IsaDora | Ultra Cover compact powder 6in1 | Polyperfluoroethoxymethoxy difluoroethyl PEG phosphate |
| POW09 | Lumene | Longwear blur | Perfluorooctyl triethoxysilane |
| POW10 | H&M | Highlight Palette (4 colors) | Polytefum (PTFE), polytef (PTFE) |
| POW11 | H&M | Face Palette (3 colors) | Polytef (PTFE) |
| POW12 | Lumene | CC color correcting powder 6 in 1 | Perfluorooctyl triethoxysilane |
| SHAV01 | Gillette | Satin Care, Pure & Delicate | PTFE |
| SHAV02b | Gillette | Satin Care, Olay, Violet Swirl Shave gel | — |
Targets of interest
A total of 39 PFASs were quantified in the present work. Since native and/or isotopically labelled standards were unavailable for some PFASs, we defined three levels of data quality. Level 1 was applied to the following 17 targets for which a native standard and exactly matched, isotopically labelled standard were available (supplier information can be found in Table S1†): linear isomers of perfluorobutanoate (PFBA), perfluoropentanoate (PFPeA), perfluorohexanoate (PFHxA), perfluoroheptanoate (PFHpA), PFOA, perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA), perfluorododecanoate (PFDoDA), perfluorohexane sulfonate (PFHxS), PFOS, perfluorooctane sulfonamide (FOSA), 1H,1H,2H,2H-perfluorooctane sulfonate (6:2 FTSA), 1H,1H,2H,2H-perfluorooctylphosphate (6:2 monoPAP), 1H,1H,2H,2H-perfluorodecylphosphate (8:2 monoPAP), bis(1H,1H,2H,2H-perfluorooctyl)phosphate (6:2/6:2 diPAP) and bis(1H,1H,2H,2H-perfluorodecyl)phosphate (8:2/8:2 diPAP). Level 2 was given to the following 13 targets for which a native standard was available but not an exactly matched isotopically labelled standard: perfluorotridecanoate (PFTrDA), dodecafluoro-3H-4,8-dioxanonanoate (ADONA), 9-chlorohexadecafluoro-3-oxanonane-1-sulfonate (9Cl-PF3ONS), 11-chloroeicosafluoro-3-oxaundecane-1-sulfonate (11Cl-PF3OUdS), perfluorobutane sulfonate (PFBS), perfluorodecane sulfonate (PFDS), perfluorooctane sulfonamidoacetic acid (FOSAA), 1H,1H,2H,2H-perfluorohexane sulfonate (4:2 FTSA), 1H,1H,2H,2H-perfluorodecane sulfonate (8:2 FTSA), 1H,1H,2H,2H-perfluorohexylphosphate (4:2 monoPAP), 1H,1H,2H,2H-perfluorododecylphosphate (10:2 monoPAP), bis(1H,1H,2H,2H-perfluorohexyl)phosphate (4:2/4:2 diPAP) and (1H,1H,2H,2H-perfluorooctyl)(1H,1H,2H,2H-perfluorodecyl)phosphate (6:2/8:2 diPAP). Finally, a data quality rating of level 3 was given to 9 targets for which native and isotopically labelled standards were unavailable and concentrations were determined semi-quantitatively using a structurally similar substance. This was only applied when there was a high degree of confidence in the identification (i.e. consistent retention times and MS/MS transitions relative to other PFAS homologues). Level 3 was applied to the following targets: perfluoroheptane sulfonate (PFHpS), perfluorononane sulfonate (PFNS), perfluoroundecane sulfonate (PFUnDS), and 6 polyfluoroalkyl phosphate diesters (4:2/6:2 diPAP, 6:2/10:2 diPAP, 8:2/10:2 diPAP, 6:2/12:2 diPAP, 8:2/12:2 diPAP, 6:2/14:2 diPAP). See Table S1† for more information.Targeted PFAS analysis
Sample extraction and targeted analysis was carried out at Stockholm University (SU). Samples were extracted using a method adapted from Powley et al. (2005).39 Briefly, 0.5 ml of 0.2 M NaOH, 5 ml methanol and 50 μl of internal standard mixture (50 ng ml−1) were added to 0.1 g of sample material. Thereafter, samples were vortexed and extracted in an ultrasonic bath at room temperature for 30 minutes. After centrifuging at 2000 rpm for 5 minutes, the supernatant was transferred to a new test tube. The extraction was repeated with 5 ml methanol and the extract centrifuged for 20 min at 3000 rpm, after which it was neutralized with 50 μl of 2 M HCl. The combined supernatants from both extractions were concentrated under a gentle stream of nitrogen to about 1 ml. 500 μl of the concentrated extract was transferred to a 1.5 ml Eppendorf tube containing 25 mg graphitized carbon (Supelclean ENVI-carb) and 50 μl glacial acetic acid, vortexed and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes before transferring the supernatant to a new Eppendorf tube to which 50 μl of recovery internal standards (20 ng ml−1) and 500 μl of 4 mM aqueous NH4OAc solution was added. The samples were stored in the freezer until the day of analysis. Before injection, the samples were vortexed, centrifuged and transferred to a micro vial.
000 rpm for 10 minutes before transferring the supernatant to a new Eppendorf tube to which 50 μl of recovery internal standards (20 ng ml−1) and 500 μl of 4 mM aqueous NH4OAc solution was added. The samples were stored in the freezer until the day of analysis. Before injection, the samples were vortexed, centrifuged and transferred to a micro vial.
Extracts were injected (5 μl) onto an Acquity UPLC (Waters Corp., Milford, MA) equipped with BEH C18 guard (5 × 2.1 mm, 1.7 μm particle size) and analytical (50 × 2.1 mm, 1.7 μm) column operated at 40 °C. Mobile phase composition and details about gradient and flow rate can be found in Tables S2 and S3.† Detection of PFASs was carried out using a triple quadrupole mass spectrometer (Xevo TQ-S, Waters Corp, Milford, MA) operated in negative electrospray ionization mode according to a method reported by Gebbink et al.40 Further information on MS parameters and MS transitions can be found in Table S1.† Quantification of individual PFASs (39 targets) was carried out using linear calibration curves (1/x weighting) ranging from 0.008 to 150 ng ml−1.
In cases where a target exceeded the concentration of the highest point in the calibration curve (some mono- and diPAPs in samples FOUN01, FOUN02 and FOUN03), the respective extracts and blanks were subjected to three levels of dilution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 1
5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) depending on the analyte concentration followed by fortification of internal standard (10 ng) and re-analysis on LC-MS/MS. The addition of internal standards to the extracts (versus to the sample) accounts for matrix effects in the quantification; however, losses during the extraction procedure are not tracked by this approach. Generally, the concentration of an analyte in the final extract can be adjusted either by reducing the amount of material subjected to extraction or by diluting the extract. However, in the case of samples FOUN01, FOUN02 and FOUN03, the concentrations were too high to find a balance of sample amount and final volume at which a reasonable amount of internal standard added to the sample would still be detectable in the diluted extract. Therefore, the concentrations reported for PAPs in those three samples are not corrected for procedural losses. Thus, these not-corrected concentrations are directly comparable to EOF concentrations, which also return concentrations in the sample extract rather than in the neat sample, and can be used directly for mass balance calculations.
500) depending on the analyte concentration followed by fortification of internal standard (10 ng) and re-analysis on LC-MS/MS. The addition of internal standards to the extracts (versus to the sample) accounts for matrix effects in the quantification; however, losses during the extraction procedure are not tracked by this approach. Generally, the concentration of an analyte in the final extract can be adjusted either by reducing the amount of material subjected to extraction or by diluting the extract. However, in the case of samples FOUN01, FOUN02 and FOUN03, the concentrations were too high to find a balance of sample amount and final volume at which a reasonable amount of internal standard added to the sample would still be detectable in the diluted extract. Therefore, the concentrations reported for PAPs in those three samples are not corrected for procedural losses. Thus, these not-corrected concentrations are directly comparable to EOF concentrations, which also return concentrations in the sample extract rather than in the neat sample, and can be used directly for mass balance calculations.
The procedural blanks that were prepared and processed independently along with all samples did not show detectable contamination for any target PFAS. Therefore, the LODs were determined using the concentration obtained from the lowest calibration point with a signal to noise ratio of at least 3 and converted to w/w units using an average sample weight. LODs are summarized in Tables S8 and S9.† In case of extract dilution, the dilution factor has to be taken into account for the calculation of the LOD for the respective sample.
Analysis of total fluorine and extractable organic fluorine
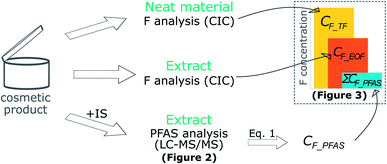
TF and EOF measurements were carried out at SU using a Thermo-Mitsubishi combustion ion chromatograph (CIC). Sample extracts (100 μl) were placed in a ceramic sample boat containing glass wool for better dispersion of the fluids while neat CP material was weighed directly into the sample boat. All boats were baked prior to sample combustion to minimize background contamination. The samples were combusted slowly in a combustion furnace (HF-210, Mitsubishi) at 1100 °C under a flow of oxygen (400 l min−1) and argon mixed with water vapor (200 l min−1) for approximately 5 minutes. Combustion gases were absorbed in MilliQ water during the entire length of the combustion process using a gas absorber unit (GA-210, Mitsubishi). An aliquot of the absorption solution (18 or 200 μl, depending on sample concentration) was injected onto an ion chromatograph (Dionex Integrion HPIC, Thermo Fisher Scientific) equipped with an anion exchange column (Dionex IonPac AS19 2 × 50 mm guard column and 2 × 250 mm analytical column, 7.5 μm particle size) operated at 30 °C. Chromatographic separation was achieved by running a gradient of aqueous hydroxide mobile phase ramping from 8 mM to 60 mM at a flow rate of 0.25 ml min−1. Fluoride was detected using a conductivity detector.Sample sizes were approximately 5 mg of neat CP material for TF analysis (i.e. direct combustion; Fig. 1) and 0.05–0.8 g of CP material for EOF (i.e. combustion of extract; Fig. 1) depending on the expected fluorine concentration. Samples intended for EOF analysis were extracted using a similar procedure as for targeted analysis described above but without addition of internal standards. The final volume of the extract was adjusted by evaporation of the solvent to about 2 ml, with the exception of high concentration samples which were kept at a final volume of 10 ml. In both cases, extraction blanks of the same final solvent volume were analyzed together with the sample extracts. More information about blank monitoring and quality control procedures can be found in the QA/QC section.
Quantification of TF and EOF was carried out using a linear six-point calibration curve of PFOS ranging from 0.5 to 100 μg ml−1 (r2 > 0.999). EOF extract concentrations were adjusted by dilution with methanol or solvent evaporation under nitrogen to fit within the concentration range of the calibration curve. The LOD for TF measurements was calculated from three times the average area obtained from instrumental blanks (n = 3) and converted using an average sample amount (3 mg) which resulted in 91.1 μg g−1. TF method blanks were below 1% of sample concentrations, and therefore TF measurements were not blank-corrected. For EOF measurements, LODs were calculated using three times the average signal of the procedural blank (n = 3) converted with the corresponding calibration curve and an average final extract volume (2 ml) and sample amount (0.5 g). LODs for EOF measurements were determined for every batch of measurements and varied depending on the inter-day variability of the instrumental response and blank level and ranged from 1.02 to 6.65 μg g−1. All EOF concentrations were blank corrected.
Fluorine mass balance calculations
Fluorine mass balance calculations were carried out by converting the concentration for a given PFAS measured by LC-MS/MS (CPFAS; ng PFAS per g) to its corresponding fluoride concentration (CF_PFAS; ng F per g) using the following equation:| CF_PFAS = nF × AF/MWPFAS × CPFAS | (1) |
| CF_EOF = ΣCF_PFAS + CF_extr.unknown | (2) |
The total fluorine concentration (CF_TF; ng F per g) as measured directly by CIC equals the sum of CF_EOF and the total non-extractable fluorine concentration (CF_non extr.; ng F per g), as shown in eqn (3).
| CF_TF = CF_EOF + CF_non extr. | (3) |
QA/QC
For individual PFASs measured by LC-MS/MS, triplicate spike/recovery experiments were performed by fortifying a PFAS-free cosmetic (FOUN07) at two fortification levels (1 and 48 ng). Precision was assessed by determining the relative standard deviation of the replicates while accuracy was calculated by comparing measured concentrations to theoretical values. Separate spike/recovery experiments were performed to validate EOF measurements by CIC. These involved fortifying both blanks and cosmetic samples with (a) 7.6 μg PFOS (n = 3), (b) 5 μg of NaF (n = 3) and (c) 7.6 μg of PFOS and 5 μg of NaF (n = 3). In addition to assessing accuracy and precision of the method for EOF, these experiments served to evaluate whether inorganic fluorine (NaF) was removed during the extraction procedure. The accuracy and precision of TF measurements were assessed through triplicate combustions of a certified reference material (CRM) (BCR®-461, fluorine in clay). In addition to the aforementioned method validation, three extraction blanks were processed along with every batch, and QC standard and blank injections were performed intermittently to assess instrument drift and carryover, respectively (both LC-MS/MS and CIC). Intra-sample variability of TF and EOF was assessed by analyzing 20% of samples in triplicate (CIC). As a final validation of the method, 18 unfortified samples were re-analyzed for 11 PFASs by IVL Swedish Environmental Institute (IVL) and compared to results generated by SU.Results and discussion
Targeted PFAS analysis
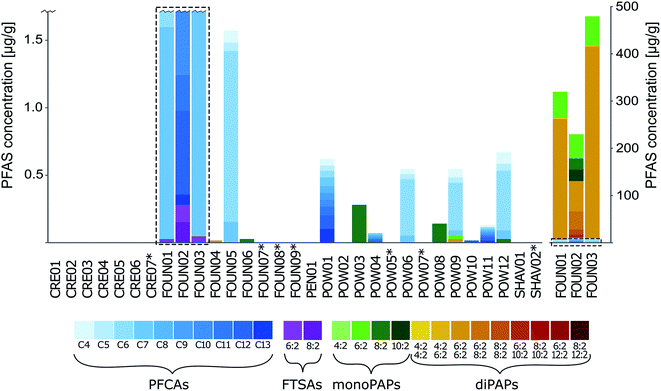
Method accuracy, as assessed through spike/recovery experiments, ranged from 71 to 126% for most PFASs (Table S4†). The exceptions were for PFHpA, 9Cl-PF3ONS, 11Cl-PF3OUdS, 4:2 FTSA, 8:2 monoPAP and 8:2/8:2 diPAP, which all had higher recoveries (up to 280%) and PFTrDA, FOSAA and 6:2/8:2 diPAP which displayed lower recoveries (as low as 29%), likely due to matrix-induced ionization effects (since qualifier ions displayed similar recoveries) which were not accounted for due to the absence of exactly-matched isotopically labelled standards. Nevertheless, most substances displayed good precision (average RSD of 24%) at both fortification levels (Table S4†). Results of the inter-laboratory comparison on a subset of 18 samples (unfortified) revealed good agreement between labs, with a coefficient of determination of 0.9605 for 11 targeted PFAS measurements (Fig. S1†).In total, 16 out of 31 samples contained measurable concentrations of at least one PFAS (see Fig. 2, Tables S7 and S8† for a full list of concentrations). Foundation and powder products contained up to 25 out of 39 monitored PFASs above detection limits, whereas samples of cream, pencil and shaving foam did not contain detectable concentrations of targeted PFASs. Samples not listing fluorinated ingredients were below LOD for all PFASs. The highest detection frequency was observed for short-chain PFCAs (PFHpA 39%, PFBA 35%, PFHxA 32%, PFPeA 29%). Six long-chain PFCA homologues were detected above the LOD (in order of decreasing detection frequency: PFUnDA > PFOA = PFDA = PFDoDA > PFNA > PFTrDA). Σ10PFCA concentrations ranged from <LOD to 9220 ng g−1 for foundations and from <LOD to 679 ng g−1 for powders and eye shadows. Although PFCAs were the most frequently detected compounds, some mono- and diPAPs were detected in far higher concentrations (up to 405 μg g−1). The dominant chain lengths for PAPs were 6:2 and 8:2, albeit homologues from 4:2 up to 12:2 were also detected. Σ14PAP concentrations ranged from <LOD to 471 μg g−1 for foundations and from <LOD to 282 ng g−1 for powders and eye shadows. Additionally, 6:2 and 8:2 FTSA were detected in three (FOUN01, FOUN02 and FOUN03; 21.9 to 132 ng g−1) and one (FOUN03, 156 ng g−1) foundation products, respectively. No perfluoroether carboxylic acids, PFSAs or related precursors were detected above LODs in any sample.
Three samples (FOUN01, FOUN02 and FOUN03) contained particularly high concentrations of PAPs and PFCAs (see Fig. 2), with Σ39PFAS concentrations of 319, 229 and 479 μg g−1, respectively. The major PFASs in these samples were 6:2/6:2 diPAP (256, 63.7 and 405 μg g−1, respectively) and 6:2 monoPAP (55.5, 50.4 and 62.0 μg g−1, respectively), accounting for 98% (FOUN01 and FOUN03) and 50% (FOUN02) of Σ39PFAS. The finding of PAPs in these samples is in agreement with the ingredients lists, which specify ammonium C6-16 perfluoroalkylethyl phosphate (FOUN01 and FOUN03) and C9-15 fluoroalcohol phosphate (FOUN02) as intentionally added components (INCI nomenclature). The co-occurrence of PFCAs (Σ10PFCA concentrations 4890, 9220 and 8480 ng g−1 respectively) and FTSAs (Σ3FTSA concentrations of 21.9, 288 and 60.6 ng g−1, respectively) in these samples is, however, more likely due to impurities in the technical mixture of PAPs or degradation of the active ingredients, since they are found in relatively low concentrations. Several other studies have demonstrated the presence of PFCAs as impurities in products treated with fluorotelomer-based substances.41–45 Our findings of PFCAs in cosmetics were in good agreement with a previous study by Fujii et al. (2013) who reported Σ9PFCA concentrations of up to 5900 ng g−1 in foundations collected from the Japanese market in 2009 and 2011.22 However, the Japanese products displayed a slightly different homologue pattern with consistently higher concentrations of PFOA compared to PFHxA. The overall higher concentrations and detection frequency of PFHxA in this study probably reflect the industrial transition from C8 to C6 fluorotelomer-based PFASs occurring in the period between when the two studies were conducted. Thus, the homologue pattern of PFASs in cosmetics appear to follow the same trend as treated textiles and papers where recent studies typically report the highest concentrations and detection frequency for C6 compounds.37,38,46
Fluorine mass balance and unidentified PFASs
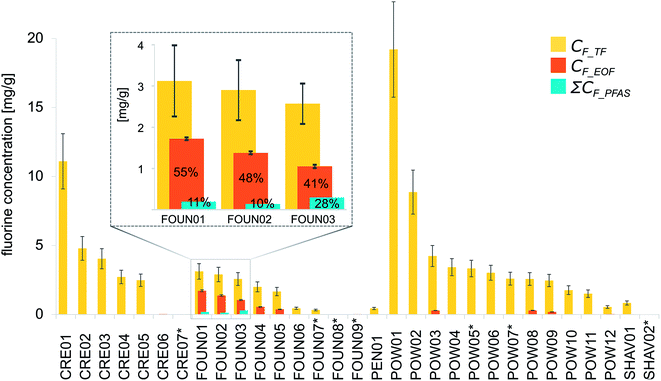
CIC spike/recovery experiments involving samples fortified with different combinations of PFOS and NaF (see method section for details) revealed that inorganic fluorine was effectively removed during the extraction procedure (i.e. fluorine was not detected in samples fortified only with NaF). This was remarkable as previous studies had found it necessary to use a solid phase extraction step for efficient removal of inorganic fluorine.25,31 Furthermore, PFOS fortified into foundation or blanks revealed reasonable accuracy and precision, given that no internal standard was used to account for losses during the extraction procedure (recovery of 69 ± 14% RSD, n = 3 replicates for spiked foundation and blanks, see Table S6†). Validation of TF measurements via direct combustion of the CRM (BCR®-461, fluorine in clay) revealed excellent agreement between measured (565 ± 15 mg kg−1, n = 3) and certified concentrations (568 ± 60 mg kg−1). Finally, intra-sample variability (assessed via triplicate measurements of 20% of samples) averaged 10% for TF and 3% for EOF measurements. The larger intra-sample variability for TF compared to EOF measurements may be attributed in part to the uncertainty of weighing small amounts of neat product material and potential heterogeneity of the small aliquots.Twenty-three out of the 24 samples listing fluorinated ingredients produced TF measurements >LOD, while EOF concentrations were above LOD for 17 samples (Fig. 3 and Table S9†). Overall, TF and EOF content showed high variability within each product category. The highest TF concentrations were found in powders (547–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 μg g−1), closely followed by creams (<LOD – 11
200 μg g−1), closely followed by creams (<LOD – 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 μg g−1) and foundations (326–3120 μg g−1). Pencil and shaving foams displayed lower TF concentrations (438 and <LOD – 837 μg g−1, respectively). As expected, EOF concentrations were consistently lower than TF, accounting for 0–55% of the total fluorine (averaging 9%). In contrast to TF concentrations, the highest EOF concentrations were measured in foundation samples (1.29–1720 μg g−1). Comparatively low EOF concentrations were observed in powders (<LOD – 296 μg g−1), creams (<LOD – 31.9 μg g−1) and shaving foams (<LOD – 3.53 μg g−1). No EOF was detected in the pencil sample.
100 μg g−1) and foundations (326–3120 μg g−1). Pencil and shaving foams displayed lower TF concentrations (438 and <LOD – 837 μg g−1, respectively). As expected, EOF concentrations were consistently lower than TF, accounting for 0–55% of the total fluorine (averaging 9%). In contrast to TF concentrations, the highest EOF concentrations were measured in foundation samples (1.29–1720 μg g−1). Comparatively low EOF concentrations were observed in powders (<LOD – 296 μg g−1), creams (<LOD – 31.9 μg g−1) and shaving foams (<LOD – 3.53 μg g−1). No EOF was detected in the pencil sample.
FOUN01, FOUN02 and FOUN03, which displayed the highest Σ39PFAS concentrations, also showed high TF (3120, 2900 and 2570 μg g−1 respectively) and EOF concentrations (1720, 1380 and 1050 μg g−1 respectively). In these samples, ΣCF_PFAS accounted for 11, 10 and 28% of CF_EOF respectively, which in turn accounted for 55, 48 and 41% of CF_TF (Fig. 3). According to eqn (2), the total concentration of unidentified, extractable organic fluorine CF_extr.unknown amounts to 1520, 1240 and 754 μg g−1 respectively. Here it is germane to note that for these three samples, concentrations of PAPs (which represented the major fraction of characterized EOF) were not corrected for procedural losses (see explanation in Methods section). Consequently, ΣCF_PAP (accouting for 98% of ΣCF_PFAS) and CF_EOF (which also is not corrected for procedural loss) are directly comparable and indicate the presence of non-targeted PFASs or other fluorinated compounds.
For the remaining samples, the fraction of CF_EOF accounted for by ΣCF_PFAS was negligible (≤1.3%), with remaining CF_extr.unknown of up to 552 μg g−1. As in these cases all PFAS concentrations were adjusted for extraction losses while EOF concentrations are not, the gap between CF_EOF and ΣCF_PFAS (CF_extr.unknown) should be considered conservative (i.e. underestimated). However, as the listed fluorinated ingredients in these samples (perfluorooctyl triethoxysilane, trifluoropropyl dimethiconol, perfluoropropyl dimethicone, and polymers polytetrafluoroethylene (PTFE, also known as Teflon, polytefum or polytef), polyperfluoromethylisopropyl ether, trifluoroacetyl tripeptide-2 and polyperfluoroethoxymethoxy difluoroethyl PEG phosphate) were not quantified due to lack of analytical methods and standards, the significance of CF_extr.unknown in these samples remains unclear. Notably, the four samples listing perfluorooctyl triethoxysilane (FOUN05, POW06, POW09 and POW12) all contained C4–C7 PFCAs, dominated by the C6 homologue. These PFCAs are presumably impurities or degradation products of the 6:2 fluorotelomer moiety of the perfluorooctyl triethoxysilane, given that they did not appear on ingredients lists. Overall these data indicate that fluorine-containing ingredients do not necessarily account for all of the measured EOF, and that other PFASs not included in ingredient lists may be important.
All samples listing PTFE as ingredient had high TF concentrations (n = 9, 837–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 μg g−1), whereas EOF could only be detected in 5 of the PTFE-containing samples in low concentrations (<LOD – 12.3 μg g−1), suggesting that PTFE is not extracted with methanol. Samples containing other polymers, namely polyperfluoroethoxymethoxy difluoroethyl PEG phosphate (FOUN04, POW03 and POW08) and polyperfluoromethylisopropyl ether (CRE02 and CRE03), also showed high TF (2000–4240 μg g−1 and 4040–4790 μg g−1 respectively) and low EOF contents (289–552 μg g−1 and <LOD respectively). Whereas PTFE-containing samples contained low levels of PFCAs (C4–C13), and polyperfluoroethoxymethoxy difluoroethyl PEG phosphate-containing products contained low levels of PAPs (8:2 mono and 6:2/6:2 diPAP) as residues, no targeted PFASs were detected in polyperfluoromethylisopropyl ether containing products. Interestingly, only one of the seven supposedly fluorine-free samples (CRE07) was below LOD for both TF and EOF. Five of the CPs not listing any fluorinated ingredients had detectable TF levels (<LOD – 3330 μg g−1), and three samples had detectable EOF content (<LOD – 30.2 μg g−1).
200 μg g−1), whereas EOF could only be detected in 5 of the PTFE-containing samples in low concentrations (<LOD – 12.3 μg g−1), suggesting that PTFE is not extracted with methanol. Samples containing other polymers, namely polyperfluoroethoxymethoxy difluoroethyl PEG phosphate (FOUN04, POW03 and POW08) and polyperfluoromethylisopropyl ether (CRE02 and CRE03), also showed high TF (2000–4240 μg g−1 and 4040–4790 μg g−1 respectively) and low EOF contents (289–552 μg g−1 and <LOD respectively). Whereas PTFE-containing samples contained low levels of PFCAs (C4–C13), and polyperfluoroethoxymethoxy difluoroethyl PEG phosphate-containing products contained low levels of PAPs (8:2 mono and 6:2/6:2 diPAP) as residues, no targeted PFASs were detected in polyperfluoromethylisopropyl ether containing products. Interestingly, only one of the seven supposedly fluorine-free samples (CRE07) was below LOD for both TF and EOF. Five of the CPs not listing any fluorinated ingredients had detectable TF levels (<LOD – 3330 μg g−1), and three samples had detectable EOF content (<LOD – 30.2 μg g−1).
One of the study limitations was that some of the listed fluorinated ingredients (e.g. fluorinated silanes, polymeric substances) were not quantified due to the lack of MS-based methods and/or authentic standards. In those cases, the mass balance gap between Σ39PFASs and EOF may be explained by the active ingredient. However, some of these CPs contained PFASs impurities which are not likely to originate from the listed fluorinated ingredient and might therefore indicate the presence of other PFAS or PFAS precursors in these products. Samples in which the active ingredient was quantified (i.e. PAPs) still showed a large gap between Σ39PFASs and EOF, hinting at the presence of other fluorinated substances that were not included in the targeted analysis. Similarly, TF and EOF concentrations were significantly different in all samples with the EOF content only accounting 9% of TF on average. This difference reflects on one hand the extraction efficiency of low molecular substances (e.g. PAPs) and on the other hand the presence of non-extractable substances such as polymers or inorganic fluorine (e.g. fluoride or fluorphlogopite).
Implications for human exposure and environmental emissions
This study demonstrates that PFCAs of various chain-lengths are present in CPs as impurities or degradation products of other PFASs which are added as active ingredients. Dermal exposure to PFCAs has, thus far, been considered negligible relative to contributions from dietary intake, drinking water and ingestion of house dust according to current human exposure assessments.47,48 However, these exposure assessments have not considered the potential contribution of CPs towards dermal uptake due to the lack of measurement data. Furthermore, the assessments for other types of consumer products (e.g. PFAS-treated textiles and impregnation sprays) may have underestimated the exposure by using the dermal absorption coefficients for the ammonium salt of PFOA49 without consideration of how ionization status, co-solvents etc. may affect dermal penetration. Thus, the results from this study on PFASs in CPs in combination with more recent dermal permeability studies of PFOA underscore the need to revisit the potential contribution of dermal exposure.50Considering that foundations displayed the highest detection frequency and absolute concentrations of PFASs the following calculation illustrates the potential importance of dermal exposure to PFASs. According to the US EPA exposure factors handbook the amount of foundation per application is 0.265 gram. Assuming a daily application of foundation, a body weight of 60 kg and that 30% of the applied PFOA has penetrated the skin after 10 hours (ref. 50) the estimated daily exposure to PFOA from CPs analyzed in this study would be in the range <0.006–3.1 ng per kg per day. The higher-end values indicate that frequent application of specific CPs can lead to a dermal exposure to PFOA which exceeds daily intakes of PFOA via diet (0.04–0.7 ng per kg per day) for the Swedish population.51,52 It should also be noted that these calculations do not consider indirect exposure to PFOA via metabolism of PAPs, which were present in 100-fold higher concentrations compared to PFCAs, or other potential precursors that contributed to the EOF fraction. Clearly, more research is needed to establish dermal absorption coefficients for a wider range of PFASs and application conditions and the potential transformation of PFASs by phase 1 and phase 2 enzymes and photolytic processes in or on the skin.53–55
In addition to the potential for human exposure, there may be environmental risks associated with PFASs in CPs which enter waste streams following use. It is unclear how these substances will behave during wastewater treatment. However, waste water treatment plants (WWTPs) have been identified as a significant source of PFASs, either to air, receiving water, and fields where sludge is applied as fertilizer.56,57 A recent fluorine mass balance study of Swedish WWTPs reported that 42–82% of extractable organic fluorine in sludge and 5–21% in effluent were unaccounted for. Notably, diPAPs, which are among the known ingredients in some CPs, contributed a major proportion (63%) to the ΣPFAS concentrations in sludge samples.57
Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
We would like to thank Oskar Sandblom for assistance with sample analysis. This project was funded by the Swedish Research Council Formas (Grant number 2013-00794) and the Swedish Society for Nature Conservation (SSNC).References
- E. Kissa, Fluorinated Surfactants and Repellents (2nd Edition Revised and Expanded), Marcel Dekker, 2001 Search PubMed.
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. De Voogt, A. A. Jensen, K. Kannan, S. A. Mabury and S. P. J. van Leeuwen, Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins, Integr. Environ. Assess. Manage., 2011, 7(4), 513–541 CrossRef CAS PubMed.
- J. P. Giesy and K. Kannan, Global Distribution of Perfluorooctane Sulfonate in Wildlife, Environ. Sci. Technol., 2001, 35(7), 1339–1342 CrossRef CAS PubMed.
- K. J. Hansen, L. a. Clemen, M. E. Ellefson and H. O. Johnson, Compound-Specific, Quantitative Characterization of Organic Fluorochemicals in Biological Matrices, Environ. Sci. Technol., 2001, 35(4), 766–770 CrossRef CAS PubMed.
- J. M. Conder, R. A. Hoke, W. De Wolf, M. H. Russell and R. C. Buck, Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds, Environ. Sci. Technol., 2008, 995–1003 CrossRef CAS.
- K. Steenland, T. Fletcher and D. A. Savitz, Epidemiologic Evidence on the Health Effects of Perfluorooctanoic Acid (PFOA), Environ. Health Perspect., 2010, 118(8), 1100–1108 CrossRef CAS PubMed.
- V. Barry, A. Winquist and K. Steenland, Perfluorooctanoic Acid (PFOA) Exposures and Incident Cancers among Adults Living near a Chemical Plant, Environ. Health Perspect., 2013, 121(11–12), 1313–1318 CrossRef PubMed.
- A. Winquist and K. Steenland, Modeled PFOA Exposure and Coronary Artery Disease, Hypertension, and High Cholesterol in Community and Worker Cohorts, Environ. Health Perspect., 2014, 122(12), 1299–1305 CrossRef CAS PubMed.
- A. B. Lindstrom, M. J. Strynar and E. L. Libelo, Polyfluorinated Compounds: Past, Present, and Future, Environ. Sci. Technol., 2011, 45(19), 7954–7961 CrossRef CAS PubMed.
- Z. Wang, I. T. Cousins, M. Scheringer and K. Hungerbühler, Fluorinated Alternatives to Long-Chain Perfluoroalkyl Carboxylic Acids (PFCAs), Perfluoroalkane Sulfonic Acids (PFSAs) and Their Potential Precursors, Environ. Int., 2013, 60(2013), 242–248 CrossRef CAS PubMed.
- Z. Wang, I. T. Cousins, M. Scheringer and K. Hungerbühler, Hazard Assessment of Fluorinated Alternatives to Long-Chain Perfluoroalkyl Acids (PFAAs) and Their Precursors: Status Quo, Ongoing Challenges and Possible Solutions, Environ. Int., 2015, 75, 172–179 CrossRef CAS PubMed.
- Z. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbühler, Global Emission Inventories for C4–C14 Perfluoroalkyl Carboxylic Acid (PFCA) Homologues from 1951 to 2030, Part I: Production and Emissions from Quantifiable Sources, Environ. Int., 2014, 70, 62–75 CrossRef CAS PubMed.
- Z. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbühler, Global Emission Inventories for C4-C14 Perfluoroalkyl Carboxylic Acid (PFCA) Homologues from 1951 to 2030, Part II: The Remaining Pieces of the Puzzle, Environ. Int., 2014, 69, 166–176 CrossRef CAS PubMed.
- Commission Regulation (EU), https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1534354204793%26uri=CELEX:32010R0757, No. 757/2010, (accessed Aug 15, 2018).
- Commission Regulation (EU)2017/1000, https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1528983620555%26uri=CELEX:32017R1000, (accessed Jun 14, 2018).
- OECD, Toward a New Comprehensive Global Database Pf Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of per-and Polyfluoroalkyl Substances (PFASs), 2018 Search PubMed.
- KEMI Swedish Chemical Agency, Occurrence and Use of Highly Fluorinated Substances and Alternatives, 2015 Search PubMed.
- CosIng - Cosmetics - GROWTH - European Commission http://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.simple, (accessed Aug 15, 2018).
- Regulation (EC), No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32009R1223, (accessed Jul 4, 2018).
- FDA, Federal Food, Drug and Cosmetics Act, https://www.fda.gov/cosmetics/productsingredients/ingredients/default.htm, (accessed Sep 30, 2018).
- C. Henricsson, Förekomst Av PFAS i Kosmetiska Produkter, Master Thesis, Lund University, 2017.
- Y. Fujii, K. H. Harada and A. Koizumi, Occurrence of Perfluorinated Carboxylic Acids (PFCAs) in Personal Care Products and Compounding Agents, Chemosphere, 2013, 93(3), 538–544 CrossRef CAS PubMed.
- A. Jahnke, L. Ahrens, R. Ebinghaus, U. Berger, J. L. Barber and C. Temme, An Improved Method for the Analysis of Volatile Polyfluorinated Alkyl Substances in Environmental Air Samples, Anal. Bioanal. Chem., 2007, 387(3), 965–975 CrossRef CAS PubMed.
- K. Rankin and S. A. Mabury, Matrix Normalized MALDI-TOF Quantification of a Fluorotelomer-Based Acrylate Polymer, Environ. Sci. Technol., 2015, 49(10), 6093–6101 CrossRef CAS PubMed.
- Y. Miyake, N. Yamashita, M. K. So, P. Rostkowski, S. Taniyasu, P. K. S. Lam and K. Kannan, Trace Analysis of Total Fluorine in Human Blood Using Combustion Ion Chromatography for Fluorine: A Mass Balance Approach for the Determination of Known and Unknown Organofluorine Compounds, J. Chromatogr. A, 2007, 1154(1–2), 214–221 CrossRef CAS PubMed.
- Y. Miyake, N. Yamashita, P. Rostkowski, M. K. So, S. Taniyasu, P. K. S. Lam and K. Kannan, Determination of Trace Levels of Total Fluorine in Water Using Combustion Ion Chromatography for Fluorine: A Mass Balance Approach to Determine Individual Perfluorinated Chemicals in Water, J. Chromatogr. A, 2007, 1143(1–2), 98–104 CrossRef CAS PubMed.
- S. Willach, H. J. Brauch and F. T. Lange, Contribution of Selected Perfluoroalkyl and Polyfluoroalkyl Substances to the Adsorbable Organically Bound Fluorine in German Rivers and in a Highly Contaminated Groundwater, Chemosphere, 2016, 145, 342–350 CrossRef CAS PubMed.
- E. E. Ritter, M. E. Dickinson, J. P. Harron, D. M. Lunderberg, P. A. DeYoung, A. E. Robel, J. A. Field and G. F. Peaslee, PIGE as a Screening Tool for Per- and Polyfluorinated Substances in Papers and Textiles, Nucl. Instrum. Methods Phys. Res., Sect. B, 2017, 407, 47–54 CrossRef CAS.
- L. W. Y. Yeung, Y. Miyake, S. Taniyasu, Y. Wang, H. Yu, M. K. So, G. Jiang, Y. Wu, J. Li and J. P. Giesy, et al., Perfluorinated Compounds and Total and Extractable Organic Fluorine in Human Blood Samples from China, Environ. Sci. Technol., 2008, 42(21), 8140–8145 CrossRef CAS PubMed.
- L. W. Y. Yeung, Y. Miyake, P. Li, S. Taniyasu, K. Kannan, K. S. Guruge, P. K. S. Lam and N. Yamashita, Comparison of Total Fluorine, Extractable Organic Fluorine and Perfluorinated Compounds in the Blood of Wild and Pefluorooctanoate (PFOA)-Exposed Rats: Evidence for the Presence of Other Organofluorine Compounds, Anal. Chim. Acta, 2009, 635(1), 108–114 CrossRef CAS PubMed.
- L. W. Y. Yeung, Y. Miyake, Y. Wang, S. Taniyasu, N. Yamashita and P. K. S. Lam, Total Fluorine, Extractable Organic Fluorine, Perfluorooctane Sulfonate and Other Related Fluorochemicals in Liver of Indo-Pacific Humpback Dolphins (Sousa Chinensis) and Finless Porpoises (Neophocaena Phocaenoides) from South China, Environ. Pollut., 2009, 157(1), 17–23 CrossRef CAS PubMed.
- E. I. H. Loi, L. W. Y. Yeung, S. Taniyasu, P. K. S. Lam, K. Kannan and N. Yamashita, Trophic Magnification of Poly- and Perfluorinated Compounds in a Subtropical Food Web, Environ. Sci. Technol., 2011, 45(13), 5506–5513 CrossRef CAS PubMed.
- L. W. Y. Yeung and S. A. Mabury, Bioconcentration of Aqueous Film-Forming Foam (AFFF) in Juvenile Rainbow Trout (Oncorhyncus Mykiss), Environ. Sci. Technol., 2013, 47(21), 12505–12513 CrossRef CAS PubMed.
- A. Wagner, B. Raue, H. J. Brauch, E. Worch and F. T. Lange, Determination of Adsorbable Organic Fluorine from Aqueous Environmental Samples by Adsorption to Polystyrene-Divinylbenzene Based Activated Carbon and Combustion Ion Chromatography, J. Chromatogr. A, 2013, 1295, 82–89 CrossRef CAS PubMed.
- L. W. Y. Yeung, S. J. Robinson, J. Koschorreck and S. A. Mabury, Part I. A Temporal Study of PFCAs and Their Precursors in Human Plasma from Two German Cities 1982-2009, Environ. Sci. Technol., 2013, 47(8), 3865–3874 CrossRef CAS PubMed.
- L. W. Y. Yeung, S. J. Robinson, J. Koschorreck and S. A. Mabury, Part I. A Temporal Study of PFCAs and Their Precursors in Human Plasma from Two German Cities 1982-2009, Environ. Sci. Technol., 2013, 47(8), 3865–3874 CrossRef CAS PubMed.
- A. E. Robel, K. Marshall, M. Dickinson, D. Lunderberg, C. Butt, G. Peaslee, H. M. Stapleton and J. A. Field, Closing the Mass Balance on Fluorine on Papers and Textiles, Environ. Sci. Technol., 2017, 51(16), 9022–9032 CrossRef CAS PubMed.
- L. A. Schaider, S. A. Balan, A. Blum, D. Q. Andrews, M. J. Strynar, M. E. Dickinson, D. M. Lunderberg, J. R. Lang and G. F. Peaslee, Fluorinated Compounds in U.S. Fast Food Packaging, Environ. Sci. Technol. Lett., 2017, 4(3), 105–111 CrossRef CAS PubMed.
- C. R. Powley, S. W. George, T. W. Ryan and R. C. Buck, Matrix Effect-Free Analytical Methods for Determination of Perfluorinated Carboxylic Acids in Environmental Matrixes, Anal. Chem., 2005, 77(19), 6353–6358 CrossRef CAS PubMed.
- W. A. Gebbink, A. Glynn and U. Berger, Temporal Changes (1997-2012) of Perfluoroalkyl Acids and Selected Precursors (Including Isomers) in Swedish Human Serum, Environ. Pollut., 2015, 199, 166–173 CrossRef CAS PubMed.
- S. T. Washburn, T. S. Bingman, S. K. Braithwaite, R. C. Buck, L. W. Buxton, H. J. Clewell, L. A. Haroun, J. E. Kester, R. W. Rickard and A. M. Shipp, Exposure Assessment and Risk Characterization for Perfluorooctanoate in Selected Consumer Articles, Environ. Sci. Technol., 2005, 39(11), 3904–3910 CrossRef CAS PubMed.
- M. J. A. Dinglasan-Panlilio and S. A. Mabury, Significant Residual Fluorinated Alcohols Present in Various Fluorinated Materials, Environ. Sci. Technol., 2006, 40(5), 1447–1453 CrossRef CAS PubMed.
- B. S. Larsen, P. Stchur, B. Szostek, S. F. Bachmura, R. C. Rowand, K. B. Prickett, S. H. Korzeniowski and R. C. Buck, Method Development for the Determination of Residual Fluorotelomer Raw Materials and Perflurooctanoate in Fluorotelomer-Based Products by Gas Chromatography and Liquid Chromatography Mass Spectrometry, J. Chromatogr. A, 2006, 1110(1–2), 117–124 CrossRef CAS PubMed.
- K. Prevedouros, I. T. Cousins, R. C. Buck and S. H. Korzeniowski, Sources, Fate and Transport of Perfluorocarboxylates, Environ. Sci. Technol., 2006, 40(1), 32–44 CrossRef CAS PubMed.
- S. Fiedler, G. Pfister and K.-W. Schramm, Poly- and Perfluorinated Compounds in Household Consumer Products, Toxicol. Environ. Chem., 2010, 92(10), 1801–1811 CrossRef CAS.
- R. Vestergren, D. Herzke, T. Wang and I. T. Cousins, Are Imported Consumer Products an Important Diffuse Source of PFASs to the Norwegian Environment?, Environ. Pollut., 2015, 198, 223–230 CrossRef CAS PubMed.
- D. Trudel, L. Horowitz, M. Wormuth, M. Scheringer, I. T. Cousins and K. Hungerbühler, Estimating Consumer Exposure to PFOS and PFOA, Risk Anal., 2008, 28(2), 251–269 CrossRef PubMed.
- R. Vestergren, I. T. Cousins, D. Trudel, M. Wormuth and M. Scheringer, Estimating the Contribution of Precursor Compounds in Consumer Exposure to PFOS and PFOA, Chemosphere, 2008, 73(10), 1617–1624 CrossRef CAS PubMed.
- W. J. Fasano, G. L. Kennedy, B. Szostek, D. G. Farrar, R. J. Ward, L. Haroun and P. M. Hinderliter, Penetration of Ammonium Perfluorooctanoate through Rat and Human Skin in Vitro, Drug Chem. Toxicol., 2005, 28(1), 79–90 CrossRef CAS PubMed.
- J. Franko, B. J. Meade, H. F. Frasch, M. Barbero and S. E. Anderson, Dermal Penetration Potential of Perfluorooctanoic Acid (PFOA) in Human and Mouse Skin, J. Toxicol. Environ. Health, Part A, 2012, 75(1), 50–62 CrossRef CAS PubMed.
- R. Vestergren, U. Berger, A. Glynn and I. T. Cousins, Dietary Exposure to Perfluoroalkyl Acids for the Swedish Population in 1999, 2005 and 2010, Environ. Int., 2012, 49, 120–127 CrossRef CAS PubMed.
- W. A. Gebbink, A. Glynn, P. O. Darnerud and U. Berger, Perfluoroalkyl Acids and Their Precursors in Swedish Food: The Relative Importance of Direct and Indirect Dietary Exposure, Environ. Pollut., 2015, 198, 108–115 CrossRef CAS PubMed.
- A. Pannatier, P. Jenner, B. Testa and J. C. Etter, The Skin as a Drug-Metabolizing Organ, Drug Metab. Rev., 1978, 8(2), 319–343 CrossRef CAS PubMed.
- F. Oesch, E. Fabian, K. Guth and R. Landsiedel, Xenobiotic-Metabolizing Enzymes in the Skin of Rat, Mouse, Pig, Guinea Pig, Man, and in Human Skin Models, Arch. Toxicol., 2014, 88(12), 2135–2190 CrossRef CAS PubMed.
- R. M. Sayre, J. C. Dowdy, A. J. Gerwig, W. J. Shields and R. V. Lloyd, Unexpected Photolysis of the Sunscreen Octinoxate in the Presence of the Sunscreen Avobenzone, Photochem. Photobiol., 2005, 81(2), 452–456 CrossRef CAS PubMed.
- L. Ahrens, M. Shoeib, T. Harner, S. C. Lee, R. Guo and E. J. Reiner, Wastewater Treatment Plant and Landfills as Sources of Polyfluoroalkyl Compounds to the Atmosphere, Environ. Sci. Technol., 2011, 45(19), 8098–8105 CrossRef CAS PubMed.
- L. W. Y. Yeung, U. Eriksson and A. KärrmanPilotstudie Avseende Oidentifierade Poly-Och Perfluorerade Alkylämnen i Slam Och Avloppsvatten Från Reningsverk i Sverige, Naturvårdsverket, 2016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8em00368h |
| This journal is © The Royal Society of Chemistry 2018 |