Plutonium environmental chemistry: mechanisms for the surface-mediated reduction of Pu(v/vi)†
Abstract
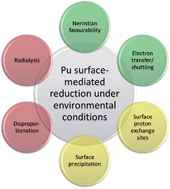
In recent decades, interest in plutonium mobility has increased significantly due to the need of the United States, as well as other nations, to deal with commercial spent nuclear fuel, nuclear weapons disarmament, and the remediation of locations contaminated by nuclear weapons testing and production. Although there is a global consensus that geologic disposal is the safest existing approach to dealing with spent nuclear fuel and high-level nuclear waste, only a few nations are moving towards implementing a geologic repository due to technical and political barriers. Understanding the factors that affect the mobility of plutonium in the subsurface environment is critical to support the development of such repositories. The importance of redox chemistry in determining plutonium mobility cannot be understated. While Pu(IV) is generally assumed to be immobile in the subsurface environment due to sorption or precipitation, Pu(V) tends to be mobile due to its relatively low effective charge and weak complex formation. This review highlights one particularly important aspect of plutonium behaviour at the mineral–water interface—the concept of surface-mediated reduction, which describes the reduction of plutonium on a mineral surface. It provides a conceptual model for and evidence supporting or refuting each proposed mechanism for surface-mediated reduction including (i) radiolysis at the mineral surface, (ii) electron transfer via ferrous iron or manganese in the mineral structure, (iii) electron shuttling due to the semiconducting properties of the mineral, (iv) disproportionation of Pu(V), (v) facilitation by proton exchange sites, (vi) stabilisation of Pu(IV) due to the increased concentration gradient within the electrical double layer, and (vii) a Nernstian favourability of Pu(IV) surface complexes and colloids. It also provides new perspectives on future research directions.


 Please wait while we load your content...
Please wait while we load your content...
