Sweat-based wearable energy harvesting-storage hybrid textile devices†
Abstract
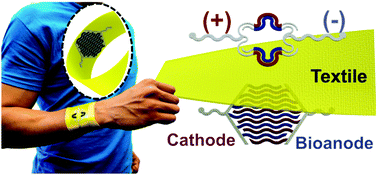
This study demonstrates the first example of a stretchable and wearable textile-based hybrid supercapacitor–biofuel cell (SC–BFC) system. The hybrid device, screen-printed on both sides of the fabric, is designed to scavenge biochemical energy from the wearer's sweat using the BFC module and to store it in the SC module for subsequent use. The BFC relies on lactate, which is oxidized enzymatically to generate electricity. The generated bioenergy is stored directly and rapidly in the printed in-plane SCs. The SC energy-storage module employs MnO2/carbon nanotube composites that offer high areal capacitance and cycling electrochemical stability. Both printed SC and BFC devices rely on optimal elastomer-containing ink formulations and serpentine structure patterns that impart a stable electrochemical performance after a variety of mechanical deformations. Such a fabrication route ensures that the energy-harvesting and storage properties of the two integrated devices are not compromised. The SC–BFC hybrid system can thus deliver stable output over long charging periods, boost the voltage output of the BFC, and exhibit favorable cycling ability. Such attractive performance, demonstrated in successful on-body testing, along with the unique architecture and low-cost scalable fabrication, make the new garment-ased hybrid energy device useful for meeting the power and mechanical resiliency requirements of wearable electronics and smart textiles.
- This article is part of the themed collection: 2018 Energy and Environmental Science HOT Articles


 Please wait while we load your content...
Please wait while we load your content...