Open Access Article
Open Access ArticlePhotoelectrochemical water splitting: an idea heading towards obsolescence?
T. Jesper
Jacobsson

Department of Chemistry, Uppsala University, Box 538, 75121 Uppsala, Sweden. E-mail: Jacobsson.jesper.work@gmail.com
First published on 30th May 2018
Abstract
The production of hydrogen from water and sunlight is a way to address the intermittency in renewable energy production, while simultaneously generating a versatile fuel and a valuable chemical feedstock. Photoelectrochemical water splitting is one possible approach to accomplish this that has been researched since the early seventies. It has for a long time held the promise of having the potential to become the best, cheapest, and most efficient way to convert solar energy into chemical energy in the form of hydrogen, but in this paper, I argue that the time window where this could have happened has now come to an end. With the rapid development of both PV-technology and earth-abundant electrocatalysis, it will be tremendously difficult, even in the best-case scenario, for a classical photoelectrochemical water splitting device to compete with what PV-driven electrolysers can already do today. This is an insight that should influence the future of solar fuel research.
Broader contextIn a time where our sources of oil are running dry, where soot from burning coal is poisoning the air, and where keeping anthropogenic climate change at bay have reached the political agenda at the highest level, the demand for a transition into a sustainable energy system has never been greater. One conceptual solution to the problem is what is known as the hydrogen economy, where hydrogen is utilized as an energy carrier, a versatile fuel, and a means to balance the inherent intermittency from renewable energy production. To come true, this vision requires sustainable hydrogen production. Given that the sun is the most abundant energy source available, a straightforward question is how to most cheaply and efficiently capture that energy and store it in hydrogen. That is the question being discussed here. |
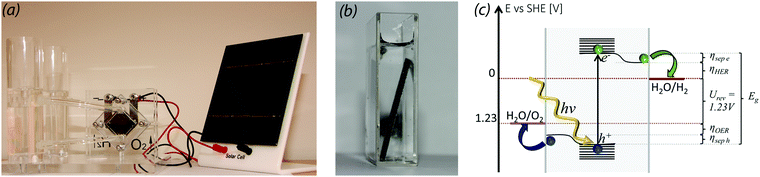
Sustainable production of hydrogen from solar energy and water is a matter of separation, transport, and transfer of photogenerated charge carriers. This requires a photoabsorber, a pair of catalysts, and current transport between them. The straightforward solution is to use a pair of electrocatalysts for electrolysis of water, a process known since 1800, with a solar cell providing the electricity, just like in the toys sold at science museums (Fig. 1a). Photoelectrochemical water splitting (PEC-WS) aims at accomplishing this cheaper and more efficiently by monolithically integrating the photoabsorber with the catalysts, like in Fig. 1b or in, for example, Nocera's artificial leaf.1 The core of this perspective is that PEC-WS no longer is a good idea, but is heading towards obsolescence due to the remarkable development of silicon PV-technology.
PEC-WS research started in the seventies with the exploration of new photoelectrode materials. From a historical perspective, this made sense. PV-technology was expensive and required advanced manufacturing processes, whereas metal oxide photoelectrodes could be deposited by simple methods and required fewer solid-state layers. The requirements of an ideal material are demanding and involve a sufficiently high band gap, band edges straddling the redox potential of water, good transport properties, high catalytic activity, and stability in the electrolyte, as well as abundance, non-toxicity, and cheapness. This is a difficult set of constraints, but the band gap requirement alone puts the single-junction PEC-WS approach at a disadvantage.
Thermodynamically, water splitting requires 1.229 V and with overpotential losses, an electrochemical bias of at least 1.5 to 2.0 V is required (Fig. 1c). For a one-cell single-junction PEC-device, this translates into a band gap of 1.9–2.3 eV or more.2 A band gap of 2.3 eV gives a maximum current density corresponding to 10% solar-to-hydrogen (STH) efficiency, whereas a PV-electrolyser based on series interconnected silicon PV-cells and a Ni-catalyst can reach over 15%.3 1.9–2.3 eV is simply too far away from the 1.35 eV for optimal power conversion for a single junction cell given the sun's spectral distribution.4 This puts PEC-WS at a disadvantage, but the real game changer is the relation between efficiency, module price, and balancing of system (BOS) costs. The record price for PV-electricity is now below 0.03 $ per kW h and is still falling,5 thus taking silicon PV towards providing some of the cheapest electricity on the planet.6 The PV-modules themselves now only constitute a fraction of the price, whereas the BOS costs, i.e. racks, electronics, land, installation, etc., can be more than 2/3 of the total cost.6 The BOS cost for a PEC-WS system is unlikely to be significantly lower than that for a corresponding PV-electrolyser, but would probably be higher as argued below. Regardless of architecture, BOS costs scale with the system footprint, and as they make up the majority of the cost, increased device efficiency is the main driver for reducing the overall price. The hydrogen from a PEC-WS module with 10% STH-efficiency would consequently be more expensive than that from a 15% efficient PV-electrolyser, even if the modules cost nothing at all. So far, no single-junction photoelectrode capable of unbiased water splitting has been demonstrated with an STH-efficiency even remotely close to 10%. The few such systems that have been demonstrated all have band gaps above 3 eV and are thus only able to utilise the UV-part of the solar spectrum, and consequently have efficiencies below 1%.7 The important thing to notice is that even if the current obstacles could be overcome, the single junction PEC-cells would still not be good enough.
The obvious workaround is tandem architectures, which have higher theoretical efficiencies than a silicon PV-electrolyser. A tandem system with a better performance/price than silicon would, however, still have a problem. Unless the only possible mechanism for charge carrier separation is semiconductor/liquid junctions, the system could in principle be converted into even better PV-cells relying on pn-junctions.2 Instead of competing with silicon PV-electrolysers, they would then compete with a better version of themselves. Only a few tandem systems have been reported exclusively relying on semiconductor/liquid junctions. The best results have been achieved with Cu2O/BiVO48 and Cu2O/WO3,9 but they are still below 1% STH-efficiency. Almost every functional water-spitting device reported in the literature does in fact utilise conventional PV-materials relying on solid-state pn-junctions,10 and thus they have the problem of competing with themselves in a PV-electrolysis architecture.2
Charge carrier separation by semiconductor/liquid junctions is appealing in its perceived simplicity. In reality it seldom functions properly, and history has demonstrated that solid-state pn-junctions can be engineered for most materials, even nanostructured ones, and be deposited at a low cost.
The other major functionality of water splitting is catalysis. The best electrocatalysts have traditionally been based on expensive noble metals, and whereas alkaline electrolysers with Ni-catalysts are reasonably cheap, they operate at 1.8–2.4 V, corresponding to 50–70% efficiency.11 This has given PEC-WS a possible advantage in its appealing simplicity if the photoabsorber also functions as a catalyst. Combining catalytic activity in an efficient photoabsorber with the right band gap, band edge matching, stability, etc., has, however, turned out to be tremendously hard. In fact, every functional water splitter has relied on additional catalysts,10 thus fundamentally utilising electrocatalysts wired to the photoabsorber, even if the wiring functionality is in the nm-range.2 Any electrocatalyst applicable in a PEC-WS configuration can in principle be deposited on a suitable substrate and wired into a PV-electrolyser. The catalysts used for PEC-cells thus not only need to be transparent and compatible with the photoabsorber, but they must also compete with the best available electrocatalysts. Recent progress has demonstrated a number of earth-abundant electrocatalysts, e.g. Ni2P/NiO12 and Ni–NiO/NiFe-LDH,13 that can operate at potentials as low as 1.5 V. They are not yet commercially available, but it is obvious that this is where we are going. Given thermodynamics and kinetic constraints, it cannot get much better, meaning that in terms of catalysis, the best a PEC-WS system can hope for is not to beat, but to match, a PV-electrolyser.
In terms of photoabsorption and catalysis, PEC-WS can thus in the best-case scenario compete with, but not beat, PV-electrolysis with respect to performance/price. Thermodynamics simply does not allow for much more. The last half century of PEC-research has, however, not reached close to the best-case scenario. There are also other, technological, advantages of PV-electrolysers, which also puts the best-case PEC-WS scenario at a disadvantage. These include higher modularity, no electrolyte related degradation of the photoabsorber, and no optical losses in the catalyst, gas bubbles, electrolyte, and electrolyte containers. The electrolyte management also gets simpler. With solar cells in one place and catalysts compactly stacked in small units elsewhere, the amount of electrolyte, tubing, and membranes can be decreased, lowering the BOS cost, as well as simplifying heat management, gas separation, and pressurization of the hydrogen compared to a PEC-WS system with the electrolyte dispersed over the entire photoabsorber area.
The time has come to address the elephant in the room and accept the inevitable. The field of PEC-WS is dying, at least in the sense of losing the potential for fulfilling its original justification. It did not solve the problem in the window of opportunity there was, whereas progress in PV-electrolysis has made even the most optimistic targets obsolete. I am claiming this, not as a sceptical outsider, but as a previous enthusiast with years of research in this endeavour, who thinks it is time to accept the inevitable and move on towards other intellectual challenges and more promising enterprises. The PEC-WS research conducted over the last few decades has resulted in much insight into, for example: heterogeneous catalysis, reaction mechanisms, charge transfer processes, semiconductor photophysics, etc. PEC research is still worth doing, but it would benefit from shifting the focus from the water splitting narrative towards the specific phenomena investigated, which could be interesting in their own right.
There is a chance that I am wrong, and I am sure not everyone will agree, but the important insight is that if PEC-WS is to have even a shadow of a chance, it will need to operate very close to its fundamental thermodynamic limits. There is simply no room for compromises – whatsoever. For the ones still clinging to the PEC-narrative, I would like to challenge you with what may be the greatest intellectual challenge in the field of solar fuels, the one of demonstrating how a large-scale PEC-WS installation could possibly give a significant advantage over a PV-electrolyser. I, and the rest of the world, are eager to listen.
Conflicts of interest
There are no conflicts to declare.References
- S. Y. Reece, J. A. Hamel, K. Sung, T. D. Jarvi, A. J. Esswein, J. J. Pijpers and D. G. Nocera, Science, 2011, 334, 645–648 CrossRef PubMed
.
- T. J. Jacobsson, V. Fjallstrom, M. Edoff and T. Edvinsson, Energy Environ. Sci., 2014, 7, 2056–2070 Search PubMed
.
- T. J. Jacobsson, V. Fjällström, M. Edoff and T. Edvinsson, Sol. Energy Mater. Sol. Cells, 2015, 138, 86–95 CrossRef
.
- C. H. Henry, J. Appl. Phys., 1980, 51, 4494–4500 CrossRef
.
- E. Bellini, PV Magazine, 2017 Search PubMed
, https://www.pv-magazine.com/2017/10/06/interview-after-saudi-arabia-how-low-can-solar-prices-go/.
- V. Shah and J. Booream-Phelps, Deutsche Bank. Industry Solar, 2015.
- F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC
.
- P. Bornoz, F. F. Abdi, S. D. Tilley, B. Dam, R. Van De Krol, M. Graetzel and K. Sivula, J. Phys. Chem. C, 2014, 118, 16959–16966 Search PubMed
.
- J. Luo, L. Steier, M.-K. Son, M. Schreier, M. T. Mayer and M. Grätzel, Nano Lett., 2016, 16, 1848–1857 CrossRef PubMed
.
- J. W. Ager, M. R. Shaner, K. A. Walczak, I. D. Sharp and S. Ardo, Energy Environ. Sci., 2015, 8, 2811–2824 Search PubMed
.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef
.
- B. You, N. Jiang, M. Sheng, M. W. Bhushan and Y. Sun, ASC Catal., 2015, 6, 714–721 Search PubMed
.
- X. Liu, X. Wang, X. Yuan, W. Dong and F. Huang, J. Mater. Chem. A, 2016, 4, 167–172 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2018 |