Universal kinetic solvent effects in acid-catalyzed reactions of biomass-derived oxygenates†
Abstract
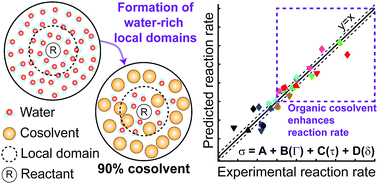
The rates of Brønsted-acid-catalyzed reactions of ethyl tert-butyl ether, tert-butanol, levoglucosan, 1,2-propanediol, fructose, cellobiose, and xylitol were measured in solvent mixtures of water with three polar aprotic cosolvents: γ-valerolactone; 1,4-dioxane; and tetrahydrofuran. As the water content of the solvent environment decreases, reactants with more hydroxyl groups have higher catalytic turnover rates for both hydrolysis and dehydration reactions. We present classical molecular dynamics simulations to explain these solvent effects in terms of three simulation-derived observables: (1) the extent of water enrichment in the local solvent domain of the reactant; (2) the average hydrogen bonding lifetime between water molecules and the reactant; and (3) the fraction of the reactant accessible surface area occupied by hydroxyl groups, all as a function of solvent composition. We develop a model, constituted by linear combinations of these three observables, that predicts experimentally determined rate constants as a function of solvent composition for the entire set of acid-catalyzed reactions.
- This article is part of the themed collection: 2018 Energy and Environmental Science HOT Articles


 Please wait while we load your content...
Please wait while we load your content...