Many ways towards ‘solar fuel’: quantitative analysis of the most promising strategies and the main challenges during scale-up†
D.
Lips‡
 a,
J. M.
Schuurmans‡
a,
J. M.
Schuurmans‡
 b,
F.
Branco dos Santos§
b,
F.
Branco dos Santos§
 *a and
K. J.
Hellingwerf§
*a and
K. J.
Hellingwerf§
 *a
*a
aMolecular Microbial Physiology Group, Faculty of Life Sciences, Swammerdam Institute of Life Sciences, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands. E-mail: f.brancodossantos@uva.nl; k.j.hellingwerf@uva.nl
bFreshwater and Marine Ecology, Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, Science Park 904, 1090 XH, Amsterdam, The Netherlands
First published on 13th October 2017
Abstract
Future global needs for liquid energy carriers, commodity chemicals and renewable materials should no longer be covered by exploration of fossilized carbon deposits. Therefore, processes are urgently needed that can replace this source of carbon for the production of these materials. The alternative route of production most often referred to is via their synthesis from CO2 (and water), using the (free) energy of sunlight. This process has been intensely studied, particularly during the past decade, and has resulted in a wide range of proposed solutions. However, with the ultimate constraint that a limited surface area will be available on our planet to catch the necessary photons, the picture is emerging showing that three approaches turn out to be most promising to achieve commercial production of this range of products. Interestingly, they all exploit living cells to facilitate formation of essential, select, carbon–carbon bonds. In one approach, photovoltaic cells provide electricity to generate hydrogen that can be used for lithoautotrophy (or: ‘chemosynthesis’) in organisms like Cupriavidus or Clostridium. An alternative approach is to use solar-driven (i.e. large-surface area) photobioreactors for the growth of engineered cyanobacteria, to carry out ‘direct conversion’ of CO2 into products like ethanol, iso-butanol, lactic acid, etc. In a hybrid derivative of these two approaches renewable (solar) electricity may be converted into monochromatic light of ∼650 nm that is optimal to drive photosynthesis in cyanobacterial photobioreactors, equipped with internal LED illumination. Here we discuss strengths and weaknesses of these three approaches, analyse the range of products for which proof-of-principle production has been demonstrated, and compare a selection of such studies with respect to efficiency and productivity of the CO2-to-product conversion. As for all approaches large-scale application is crucial, we also discuss the pitfalls and limitations of their scale-up.
Broader contextThe most often referred to route for sustainable production of liquid energy carriers, commodity chemicals, and renewable materials, is via their synthesis directly from CO2, using the energy of sunlight. This process has been intensely studied and three different approaches seem to be the most promising to achieve commercial-scale production. Their common denominator is the exploitation of living cells to facilitate the formation of target products. But while one uses solar energy directly to drive the direct conversion of CO2 into any of a wide-range of products using engineered cyanobacteria, the others require that solar energy be first used to generate electricity. The latter can then be either used to generate hydrogen, which can drive lithoautotrophic growth of organisms such as Cupriavidus or Clostridium; or be used to power narrow-band LEDs (∼650 nm) optimal to drive photosynthesis in cyanobacterial photobioreactors. Here, we analyze these approaches, and compare them with respect to their efficiency and productivity of the CO2-to-product conversion. We also discuss the possible pitfalls and limitations that each presents during the scale-up to industrial level. This report is useful to guide our efforts in accelerating the development of the much-needed alternatives to the exploitation of fossilized deposits. |
Introduction
The size of the global human population and its energy consumption are expected to increase drastically by mid-century.1 Facilitating this increase while simultaneously mitigating the onset of climate change requires the deployment of carbon-neutral energy production and manufacturing processes.2 The abundant nature of solar energy deems this energy source particularly suited to power such carbon-neutral processes, as it can be used in both natural photosynthesis and in artificial variants thereof, to convert carbon dioxide to commodity chemicals, materials and/or liquid fuel.3 Here we will refer to this process as ‘solar-to-product’- (or S2P-) conversion, and the combination of products as ‘solar fuel’.Although decreased CO2 emission can be achieved through the use of completely inorganic catalysts, the formation of carbon–carbon bonds with high efficiency and specificity, which is required for many of the important materials, commodities and fuels, remains a significant challenge in such ‘chemo-catalytic’ approaches.4–7 Because of this, purely inorganic approaches for S2P-conversion of products containing (multiple) C–C bonds, are presently not economically feasible at large scale. Instead, self-replicating and self-repairing living microbial catalysts, capable of artificial/engineered photosynthesis and CO2 fixation, and already endowed with malleable enzymatic networks for the formation of complex bioproducts, currently seem better poised to constitute future solar-powered and CO2-fueled production pipelines.
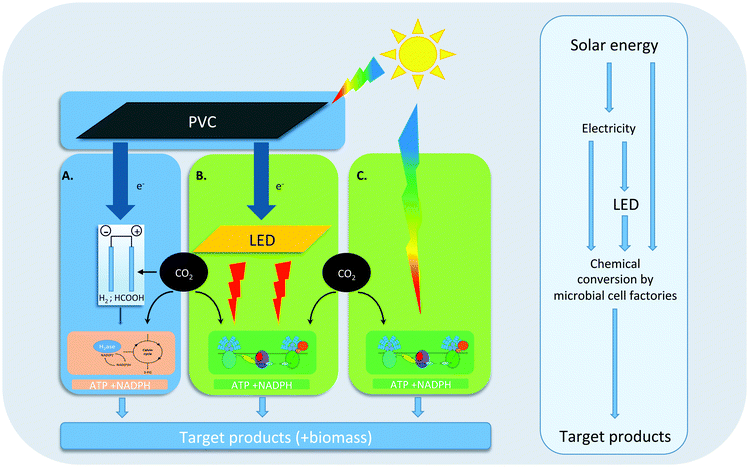
Indeed, during the past 10 years a wide variety of processes has been developed for solar fuel production in which living organisms play a key role. These processes can grossly be subdivided into three main categories (see Fig. 1 for an overview). Most recently, an approach has been advocated based on hybrid systems that use a combination of inorganic and microbial catalysts for solar fuel production. In the light-driven version of this approach (Fig. 1A), e.g. hydrogen or formic acid is either generated electrochemically at the surface of light-sensitive electrodes, or at electrodes insensitive to light, but powered by electricity from photovoltaic cells (PVC). The redox intermediate that is thus formed can subsequently be used as a source of reducing equivalents by a chemo-litho-autotrophic bacterium like Cupriavidus necator (previously known as Ralstonia eutropha8), which can be engineered to produce a variety of desired compounds. Alternatively, some microbial species, particularly acetogenic bacteria, are able to accept electrons directly from metal electrodes. Although successful scale-up for the processes summarized in Fig. 1A still remains to be shown, several proof-of-principle studies have appeared in recent years that demonstrate a variety of hybrid bio-inorganic setups for light-driven CO2 fixation into a number of fuels and chemicals.10,11 As phototrophic organisms have no role in this approach we refer to its products as ‘electrofuel’.
In the most direct S2P approach that is dependent on microbial activity (Fig. 1C), engineered cyanobacteria carry out oxygenic photosynthesis at their thylakoid membranes, to capture free energy from sunlight, which drives the synthesis of ATP and NADPH. These latter high-energy intermediates are then used in the Calvin–Benson–Bassham (CBB) cycle, in which RuBisCo catalyzes CO2 fixation for the synthesis of key metabolic intermediates, to support cellular growth. With genetic- and metabolic engineering, the metabolic network of oxygenic photosynthesis can be exploited and extended to make it produce a variety of valuable compounds from CO2, sunlight and water.12 In the past decade, proof-of-principle production data of a wide variety of compounds, by genetically engineered cyanobacteria, have been published (e.g.ref. 13; and see further below), and current efforts are aimed at increasing yields, rates and upscaling of this production, using large, closed, and sunlight-exposed surface area photobioreactors.
In a combination of these two approaches (Fig. 1B) renewably-generated electricity from PVCs, wind turbines, hydro-electric installations, etc., is converted into monochromatic light of ∼650 nm. Red light is optimal to drive photosynthesis of cyanobacteria in photobioreactors with internal illumination (in short: ‘3D photobioreactors’). Although the efficiency losses during the electricity-generation- and the subsequent light-generation step still outweigh the increased growth- and production efficacy of monochromatic ∼650 nm light versus direct sunlight, this route offers several advantages during scale-up, including the option of continuous- or L/D illumination regimes, higher biomass content, and higher-volume/smaller-area bioreactor configurations over the other two approaches.14
None of these approaches directly competes with crop production for the use of arable land, nor for the use of excessive amounts of fresh water (the main critiques on 1st and 2nd generation biofuel production processes15,16). Each approach does have its own pro's and con's, however, and their state of implementation ranges from proof-of-principle to pilot-scale production. For the successful application of any of these approaches, the volumetric productivity and the overall S2P efficiency (the conversion efficiency from solar energy into the free energy contained in the chemical bonds of the product) are, besides product cost, two of the main factors that will decide on their ultimate success. Despite the progress in this field in recent years, no quantitative analysis has yet been performed to compare the production metrics of the proof-of-principle studies of these three approaches. In this review, we will therefore discuss the benefits and downsides of all three approaches, analyze their demonstrated product ranges, and compare them quantitatively with respect to S2P conversion efficiency and production rate via analysis of a variety of published proof-of-principle studies. Since their large-scale application will be the key determinant for future commercial success, we also discuss the challenges and inherent limitations that may arise during their scale-up.
Analysis of efficiency and productivity of the ‘bio-routes’ to solar fuel
The published proof-of-principle studies that were included for the analysis that we present (see Table 1 for an overview) have primarily been selected on the basis of the availability of data required for the calculations of S2P-efficiency and -productivity. They cover a variety of representative studies, using electrochemical reactors and/or photobioreactors. For each study we have quantified the total input of light energy and output of product, to calculate S2P efficiency. In addition, we analyzed product titers over the duration of each study to derive approximate/average production rates. Enthalpy of the product (calculated from titers and heat of combustion) in relation to energy input in the form of incident light energy then allows one to calculate efficiency of product formation. Of course, the more favorable the carbon partitioning to product (i.e. %CP) is, the higher the efficiency will be. Also note that we will not discuss the biological route of ‘solar to hydrogen’,17 because it is our estimate that large-scale application of this approach will not be able to provide this energy carrier cheaper than with the combination of PVCs and an electrolyzer.18 The full derivation of all efficiency and production metrics, and their underlying assumptions, can be found in the ESI† (S1).| Organism | Product | Procedure; setup | Literature |
|---|---|---|---|
| dcc; direct conversion with cyanobacteria, LED; LED-based conversion with cyanobacteria, sef; solar-powered electrofuel setup, direct et; direct electron transfer. | |||
| S. elongatus PCC7942 | Isobutyraldehyde | dcc; 1 L-roux bottle | 24 |
| Synechocystis sp. PCC6803 | Ethanol | dcc; column reactor | 25 |
| S. elongatus PCC7942 | 2,3-Butanediol | dcc; 125 mL flask | 26 |
| Synechococcus sp. PCC7002 | Free fatty acids | dcc; 1 L-culture bottle | 27 |
| S. elongatus PCC7942 | 3-Hydroxy-propionate | dcc; 250 mL flask | 28 |
| S. elongatus PCC7942 | 1,3-Propanediol | dcc; 250 mL flask | 29 |
| Synechocystis sp. PCC6803 | Ethylene | LED; flat-panel reactor | 30 |
| C. necator H16 | Iso- & 3-rethyl-1-butanol | sef; 2-step, formate | 20 |
| C. necator H16 | Iso-propanol | sef; 2-step, hydrogen | 31 |
| C. necator H16 | PHB | sef; 2-step, hydrogen | 9 |
| C. necator H16 | Iso-propanol | sef; 2-step, hydrogen | 9 |
| C. necator H16 | Iso- & 3-methyl-1-butanol | sef; 2-step, hydrogen | 9 |
| M. barkeri | Methane | sef; integrated, hydrogen | 23 |
| S. ovata | Acetate (+PHB) | sef; integrated, direct et | 22 |
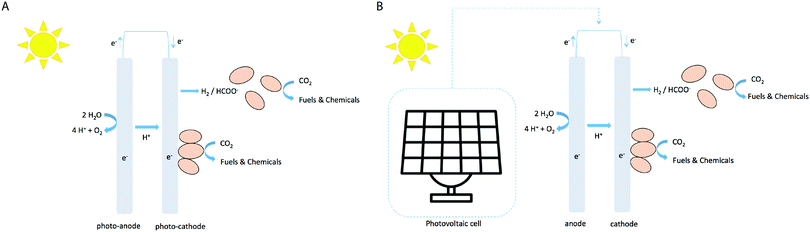
The electrofuel approach
In the electrofuel approach (named after the DOE APRA-E-funded ‘Electrofuels’ project19), artificial photosynthesis is realized by combining an inorganic light-capturing device with cells of a non-photosynthetic, litho-autotrophic, bacterium. In this approach the reactions of light harvesting and CO2 fixation are separated into subsequent steps (Fig. 1A). The light harvesting reaction can be performed by light-sensitive electrodes to either generate electrons that can directly be absorbed from the electrode by a micro-organism (with oxygen evolving at the counter electrode), or to generate diffusible reducing equivalents like hydrogen via water-splitting or formic acid via CO2 conversion (Fig. 2A). Alternatively, a PV cell first generates electricity from solar energy, which is subsequently used to generate electrons or diffusible reducing equivalents via the use of light-insensitive electrodes (Fig. 2B; e.g. in an electrolyzer). CO2 fixation in this approach can then be carried out by chemo-litho-autotrophic- or acetogenic bacteria. Below, we describe a selection of studies of electrofuel production, leveraging one of these setups in one or another format. It is relevant to note, however, that in most two-step approaches the experiments were conducted using direct current from a conventional power outlet. In those cases, the S2P efficiency is derived by virtual extension with a 20%-efficiency PVC device, to facilitate a direct comparison of these results with those of ‘direct conversion’ by cyanobacteria. An overview of relevant metrics of calculated rates and efficiencies is shown in Fig. 3.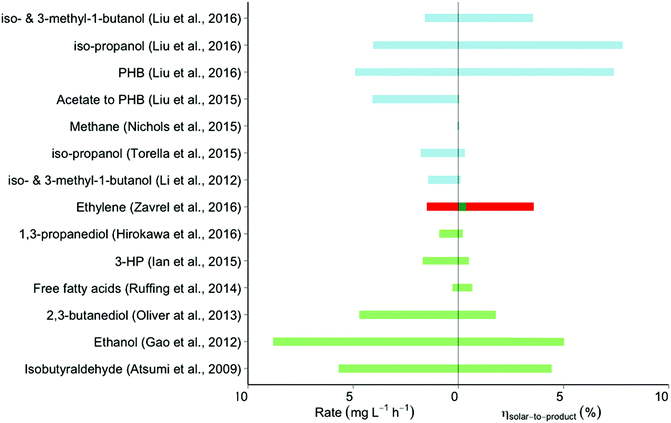 | ||
| Fig. 3 Efficiencies and rates of the relevant forms of synthesis of electrofuel and solar fuel. The overlapping colors for the ethylene S2P efficiency achieved by Zavřel et al. (2016) reflect different starting points for input energy calculation; the value indicated by the red bar results from starting with the red-wavelength photons emitted by LED lights in their study, while the green bar extends this by calculating the S2P efficiency if such LED lights were 50% efficient and powered by 20% efficiency PVCs. Further detail about the assumptions underlying the calculations is given in the text and ESI.† Color scheme: blue, direct conversion by cyanobacteria; red, LED-powered direct conversion by cyanobacteria; green, solar-powered electrofuel synthesis; PHB: poly-β-hydroxy-butyric acid. For literature: see list of references. | ||
The electrodes used in reactors for electrofuel production are often made from rare elements. Torella et al. therefore used a modified version of a previously developed ‘artificial leaf’.21 In their set-up a CoPi anode catalyzes water-splitting, in combination with a NiMoZn cathode that produces hydrogen. With a C. necator strain engineered to produce isopropanol, the authors reported the production of 0.125 mmol of this alcohol in a period of 5 days. This translates to a S2F efficiency of 0.31% and a rate of production of 1.8 mg L−1 h−1.
In contrast to this two-step approach, Liu et al. utilized an integrated setup in the form of a nanowire array consisting of TiO2/Si photoanodes and photocathodes, and the acetogen Sporumosa ovata cultivated on their surface.22 In this system, S. ovata assimilated the light-generated electrons directly from the photocathode, together with protons and CO2, to produce acetate. This intermediate substrate was then converted in a second reaction step (by Escherichia coli), into poly-β-hydroxy-butyrate (PHB), n-butanol, or any of a series of isoprenoids. Given the estimated efficiency of their initial solar-to-acetate system, and a reported acetate-to-PHB conversion efficiency by E. coli of 52%, the S2F efficiency for PHB production is 0.20% (over a 200 h period), with a rate of PHB production by E. coli of 4.1 mg L−1 h−1.
Another study by the same group also aimed for direct light-driven conversion of CO2 into product. But here, an n-TiO2-based photoanode was combined with a p-Inp/Pt photocathode to generate hydrogen, which was consumed by the methanogen Methanosarcina barkeri for the conversion of CO2 into methane.23 However, this setup produced only 68.8 nmol of methane in 72 hours, which translates into a S2P efficiency of 0.0000015% and an average methane production rate of 102 ng L−1 h−1.
Liu et al. observed that in the setup previously used by Torella et al., the formation of H2O2 was favored over the production of H2.9 Only when a sufficiently high potential was applied to the system did H2 production outweigh the toxicity effects of H2O2 sufficiently to allow growth of the cells. Substitution of the NiMoZn- by a Co–P cathode led – at lower over-potentials – to the production of H2 with negligible levels of H2O2. C. necator will synthesize PHB for intracellular carbon storage when confronted with mineral limitation in the presence of excess carbon source. In their setup, the H16 strain showed such PHB production upon nitrogen depletion, at a S2P efficiency of 7.38% and a production rate of 4.8 mg L−1 h−1. With strains engineered to produce either isopropanol or a mixture of iso-butanol and iso-pentanol, this system showed a S2P efficiency of 7.8% at a production rate of 4.01 mg L−1 h−1, and 3.3% at 1.6 mg L−1 h−1, respectively. These are the highest reported S2P efficiencies for electrofuel production reported to date.
‘Direct conversion’ by cyanobacteria
Relative to the electrofuel approach, cyanobacteria have been studied much longer, because of the interest in their exploitation as light-driven fuel- and commodity producers.32 The studies on ‘direct conversion’33 by cyanobacteria that we include in our analysis not only emphasize efficiency, but also the metabolic engineering efforts to produce novel target products. The most important aspect of these studies, nevertheless, is the metrics of conversion efficiency. These metrics can be calculated from the incident light intensity, the illuminated surface area, the volume and amount of biomass present, and the amount of product formed per unit of time (for further explanation, see ESI,† S1). Unfortunately, not all these characteristics are described in full detail in all studies considered. For some products, we therefore calculated an efficiency range based on the most plausible minimum and maximum surface area estimate. The single values presented here are the average of that range.A different but much more widely pursued biofuel target is ethanol. Its production metrics, when produced by cyanobacteria, have been significantly improved since the first attempts in the late 90s,32 as illustrated by more recent results of Gao et al.25 In the latter work, Synechocystis sp. PCC6803 was engineered to over-express pyruvate decarboxylase of Zymomonas mobilis plus a cyanobacterial alcohol hydrogenase (slr1992) at high levels. By also disrupting a competing pathway for synthesis of PHB, the authors achieved an ethanol titer of 5.5 g L−1 after 26 days of cultivation in a column photobioreactor (average rate of 8.8 mg L−1 h−1) with an incident photon flux of 55 μE s−1 m−2. Based on the estimated surface area we calculated a S2P efficiency of 5% (with an uncertainty range from 2.4% to 7.6%).
S. elongatus has also been engineered to produce 2,3-meso-butanediol. This was achieved by introducing three genes, alsS from Bacillus subtilis, alsD from Aeromonas hydrophila, and adh from Clostridium beijerincki, to form a metabolic pathway that leads to the production 2,3-meso-butanediol from pyruvate.26 At an average production rate of 4.7 mg L−1 h−1, this strain produced 2.38 g L−1 after 21 days of cultivation in a 125 mL flask filled with 25 mL of culture. This translates into an average S2P efficiency of 1.77%.
Besides alcohols and hydrocarbons, free fatty acids (FFA) can also serve as a potential fuel feedstock. Ruffing et al. achieved overproduction of FFA in Synechococcus sp. PCC 700227 by knocking out the long-chain-fatty-acid CoA ligase fadD gene and overexpressing a truncated version of E. coli's thio-esterase gene (tesA). In addition, they over-expressed RuBisCO subunits from S. elongatus PCC630. The resulting strain produced FFA at a final titer of 0.13 g L−1 after 20 days with an average rate of 3 mg L−1 h−1. The composition of these FFAs produced by S. elongatus was not reported. However, the FFA composition of cyanobacteria consists mainly of ω-16 and ω-18 fatty acids. Using palmitic acid (ω-16) as the most representative of these (heat of combustion = 10 MJ mol−1), and taking into account a culture volume of 400 mL in a 1 L bottle to calculate the relevant surface area range, and the incident photon flux of 60 μmol m−2, the average solar-to-fuel efficiency is 0.66%.
Lan et al. tested two approaches for the production of 3-hydroxypropionic acid (3HP) by Synechococcus PCC7002.28 They did so by constructing a malonyl-CoA-dependent pathway and an alanine dependent pathway, in separate strains, as well as by combing both pathways in a single strain. The genes expressed to constitute the malonyl pathway were malony-CoA reductase (mcr) from Sulfolobus tokodaii and malonate semialdehyde reductase (msr) from Metallosphaera sedula, while the -alanine dependent pathway was introduced via PEP carboxylase (ppc) from E. coli, aspartate transaminase (aspC) from E. coli, the glutamate decarboxylase adc from Aedes aegypti, and alanine amino-transferase SkPYD4 from Saccharomyces kluyveri. The best performing strain contained both pathways, and produced 3-HP at a titer of 665 mg L−1 after 16 days (equivalent to a rate of 1.7 mg L−1 h−1). Based on the use of a 250 mL flask filled with 50 mL of culture and the corresponding surface area range, the average S2P efficiency is 0.5%.
More recently, Hirokawa et al. engineered S. elongatus to produce 1,3-propanediol (13PDO).29 They constructed a pathway for 13PDO production from dihydroxyacetone phosphate (DHAP), in a four-step conversion process. First, DHAP was converted into glyercol-3-phosphate by glycerol-3-phosphate dehydrogenase GPD1, which was subsequently dephosphorylated by the phosphatase HOR2 (both enzymes from Saccharomyces cerevisiae). Glycerol was then converted to 3-hydroxypropionaldehyde by the glycerol dehydratase DhaB from Klebsiella pneumoniae, and then by an aldehyde reductase from E. coli into 13PDO. Using this pathway, they achieved a 13PDO production of 0.288 g L−1 after 14 days, with an average production rate of 0.9 mg L−1 h−1. With the incident photon flux (100 μmol s−1 m−2 and a culture volume of 50 mL in a 250 mL Erlenmeyer flask), the average S2F efficiency is 0.19%.
Two-step conversion with cyanobacteria
The third form of S2P production is essentially a hybrid form of the electrofuel and the ‘direct conversion’ approach. It is based on capturing solar (renewable) electricity, which powers monochromatic LEDs that illuminate a culture of cyanobacteria with ∼650 nm light (Fig. 1B). Use of light of this wavelength optimizes absorbance by the cyanobacterial pigments, minimizes energy dissipation, and has been shown to allow maximal – if not higher – growth rates as compared to the use of white light (which may be due to decreased photoinhibition by red-as compared to blue light).36,37 Moreover, by using LED-based illumination one can: (i) use a dynamic light regime optimally adjusted to the economics of electricity generation and (ii) optimize bioreactor configuration to path lengths of the incident light not longer than a few cm.Along these lines, Zavřel et al. engineered Synechocystis sp. PCC6803 for the production of ethylene and analyzed the S2F productivity and efficiency of producing this gaseous (commodity) product from light energy.30 They assessed two strains that both expressed the ethylene-forming enzyme (EFE) from Pseudomonas syringe, carrying this gene either on a plasmid or with two copies integrated into its genome. The authors then used a flat-panel chemostat setup, and evaluated the impact of light intensity on ethylene production by using a variety of LED-powered red light intensities ranging from 50 to 800 μE s−1 m−2. The best-performing strain showed the highest S2P efficiency at the lowest light intensity tested (50 μE s−1 m−2). Based on an ethylene production rate of 0.24 mmol g−1 DW h−1 (1.5 mg L−1 h−1), the reactor geometry38 and the available amount of light energy, the resulting S2P efficiency was 3.58%. This number is lower than the efficiency of 7.37% reported by Zavrel et al. in ref. 30. However, the calculation of Zavřel et al. only used the light energy absorbed by Synechocystis as the total input energy, while our calculation looks at the light energy striking the relevant surface area of the bioreactor and thus includes both absorbed and non-absorbed light energy. Note that the resulting efficiency of 3.58% represents the conversion of monochromatic LED light into product. Assuming conversion efficiencies of ∼20% and ∼50% for the solar-to-electricity and electricity-to-LED conversion steps,39,40 the theoretical S2P efficiency with solar energy as the starting point would be determined at 0.36%. Previous experiments have already shown that the biomass yield of Synechocystis on monochromatic red light is 1.97 times the biomass yield on photons from full-spectrum white light (∼solar light).41 One could use this ratio to scale the previously calculated S2P efficiencies for the studies with cyanobacteria in which white light was used in order to calculate theoretical LED-to-product efficiencies (see Table S3, ESI†). Of course, the solar-to-electricity and electricity-to-LED conversion losses would outweigh the near two-fold increase in light-to-product efficiency, resulting in lower overall S2P efficiencies. These lower efficiencies, however, may be at least partially compensated for by scale-up benefits, as described below. In summary, almost all studies included here fall within a range of 0–5% in terms of solar-to-product efficiency. Only recently did the use of a redox carrier with low toxicity in the electrofuel approach by Liu et al.9 allow for production of isopropanol and PHB, with engineered C. necator strains, at S2P efficiencies of 7.80% and 7.37%, respectively – well above the highest efficiencies reported for a system based on the use of cyanobacteria. These latter systems, however, deliver higher production rates (e.g. 8.8 mg L−1 h−1 and 5.7 mg L−1 h−1) by Gao et al. and Atsumi et al., versus 4.9 mg L−1 h−1 and 4.1 mg L−1 h−1 by Liu et al. (for isopropanol and PHB, respectively).
The higher efficiency of the electrofuel approach may have a basis in the intrinsic maximal efficiency of the partial processes that constitute this approach (Table 4). Whether the corresponding lower rate of production is a trade-off of this, or is due to e.g. a stringent kinetic limitation (e.g. in the rate of mass transfer from the gas to liquid phase) or the technical arrangement in which it was measured, still remains to be decided.
Versatility of product synthesis in chemo-litho-autotrophs versus cyanobacteria
To compare the range of products that can currently be produced via the S2P approaches described here, we made an inventory of the literature describing proof-of-principle metabolic engineering studies based on: (i) organisms used in electrofuel setups (in which the use of acetogens and C. necator dominates) and (ii) oxygenic photoautotrophs (i.e. cyanobacteria). The results are shown in Tables 2 and 3 for electrofuels and solar fuels, respectively. As can be seen, the cyanobacteria are currently more versatile in the range of products that they can produce. This reflects the longer period of research in which cyanobacteria have been engineered, relative to the recent emergence of engineering efforts in organisms relevant for electrofuel synthesis.| Product | Organism | Ref. |
|---|---|---|
| PHB | C. necator H16 | 9 |
| Methyl ketone | C. necator H16 | 52 |
| Isobutanol | C. necator H16 | 20 |
| Isopropanol | C. necator H16 | 53 |
| (R)-1,2-Propanediol | C. necator H16 | 54 |
| Fatty acids | C. necator H16 | 52 |
| 3-Methyl-1-butanol | C. necator H16 | 20 |
| Cyanophycin | C. necator H16 | 55 |
| 2-Methylcitric acid | C. necator H16 | 56 |
| Eugenol | C. necator H16 | 57 |
| Acetate | Sporumosa ovata | 23 |
| Ethanol | C. necator H16 | 58 |
| Butanol | Clostridium ljungdahlii | 59 |
| 2,3-Butanediol | Clostridium ljungdahlii | 60 |
| Acetone | C. aceticum | 61 |
| Butyrate | Clostridium ljungdahlii | 62 |
| Product | Organism | Ref. |
|---|---|---|
| Ethanol | Synechocystis sp. PCC6803 | 25 |
| Sucrose | S. elongatus PCC7942 | 63 |
| 2,3-Butanediol | S. elongatus PCC7942 | 64 |
| L-Lactic acid | Synechocystis sp. PCC6803 | 65 |
| D-Lactic acid | Synechocystis sp. PCC6803 | 66 |
| Glycerol | Synechocystis sp. PCC6803 | 67 |
| D-Mannitol | Synechococcus sp. PCC7002 | 68 |
| Isobutyraldehyde | S. elongatus PCC7942 | 24 |
| 3-Hydroxybutyrate | Synechocystis sp. PCC6803 | 69 |
| Isobutanol | S. elongatus PCC7942 | 24 |
| 1-Butanol | S. elongatus PCC7942 | 70 |
| 2-Methyl-1-butanol | S. elongatus PCC7942 | 71 |
| 1,2-Propanediol | S. elongatus PCC7942 | 72 |
| Glucose/fructose | S. elongatus PCC7942 | 42 |
| Acetone | Synechocystis sp. PCC6803 | 73 |
| Isopropanol | S. elongatus PCC7942 | 74 |
| Ethylene | Synechococcus sp. PCC 7942 | 75 |
| Limonene | Synechococcus sp. PCC7002 | 76 |
| Squalene | Synechocystis sp. PCC6803 | 77 |
| a-Bisabolene | Synechococcus sp. PCC7002 | 76 |
| B-Phellandrene | Synechocystis sp. PCC6803 | 78 |
| Isoprene | Synechocystis sp. PCC6803 | 79 |
| Fatty acids | Synechococcus sp. PCC7002 | 27 |
| Hydrogen | Synechococcus sp. PCC 7942 | 63 |
| Farnesene | Anabaena sp. PCC 7120 | 80 |
| Heptadecane | Synechococcus sp. NKBG15041c | 81 |
| Glucocylglycerol | Synechocystis sp. PCC6803 | 82 |
| Manyl oxide | Synechocystis sp. PCC6803 | 28 |
| 3-Hydroxypropionic acid | S. elongatus PCC7942 | 28 |
| Caffeic acid | Synechocystis sp. PCC6803 | 83 |
These product ranges expand drastically, however, when setups are considered in which the initial solar- or electrofuel product is used to feed a second microorganism like E. coli. Examples of this include the Synechococcus elongatus PCC7942 engineered by Niederholtmeyer et al. to secrete sucrose and glucose, which was subsequently used to support growth of E. coli in the absence of other carbon sources.42 Hays et al. recently cultivated the same Synechococcus strain with engineered B. subtilis and E. coli strains to demonstrate the production of α-amylase and PHB, respectively.43 With respect to electrofuel, a similar multi-organism approach has been described by Liu et al.22 The latter authors used S. ovata, interfaced with a light-sensitive nanowire array to produce acetate. The acetate was then fed to various genetically engineered strains of E. coli to produce PHB, n-butanol, and various isoprenoids. By combining organisms this way, the product range essentially becomes limitless, although the practicalities of actually implementing such ‘multi-organism production schemes’ will surely be more complicated and less efficient than using a single organism.
Efforts to engineer CO2 fixation in E. coli itself are also ongoing. Recently, this archetype workhorse of biotechnology was successfully engineered to express a non-native CBB cycle, resulting in the synthesis of various biomass constituents directly from CO2.44 Although this strain still required exogenously supplied organic compounds for the supply of reducing power, one might imagine such a strain interfaced with an electrofuel setup, or equipped with an uptake hydrogenase for growth and product formation, powered by solar or other forms of renewable energy. This would also vastly increase the accessible product range, yet with the use of a single organism.
Prospects and hurdles in future scale-up and downstream processing
For practical application and commercial success, the ability to scale-up the proof-of-concept production processes described in the studies analyzed in this review, and product recovery is essential. Their prospects and hurdles are discussed below.Optimal design of closed, large-scale photobioreactors depends, amongst many other factors like light-reflection, solar heating, turbulence, self-shading, etc., on geographic location (e.g.ref. 14). The latitude selected may affect the available light dose up to three-fold. The selected location may therefore ask for genetically-engineered adjustment of the production organism in terms of e.g. antenna cross-section and thermal stability. The former is directly related to the degree of light penetration into the reactor and the required degree of turbulence. However, because of the dilute nature of solar radiation these closed bioreactors will have to be very large, and hence their materials costs and lifetime are as important as their optimal design. Glass-based tubular reactors47 and vertically placed plastic bags48 are among the promising design strategies.
Significantly, optimization of reactor performance is often paralleled by increased reactor costs.49 For some applications a uni-species biofilm needs to attach to one of the electrodes, which in our view represents an extreme challenge for large-scale application. Indirect electron transfers via (a) soluble redox carrier(s) may therefore be better suited for large-scale electrofuel systems. These carriers need to be derived at minimal cost and without adverse side effects for the production host. Considering the recent progress in increased efficiency and scale-up of electrolysers for hydrogen,51 this latter redox carrier may become the preferred choice, although it has low solubility in water, which restricts the mass transfer rate from the gas phase to the microbial catalysts in the liquid phase, and hence productivity. Its use also poses safety hazards regarding flammability, particularly in those applications that rely on respiratory metabolism. Formic acid, the second best, is well soluble and poses less safety hazards, and recently it was shown that also with the Fe2+/Fe3+ couple significant production rates can be achieved in the electrofuel approach.49
To simplify scale-up of the production of electrofuel, Giddings et al. tested two proof-of-principle reactor designs, configured so as to avoid the use of a potentiostat or the requirement of a separator membrane to prevent oxygen diffusion (to prevent anode-produced oxygen from consuming electrons a-biotically at the cathode and interacting with oxygen-sensitive microbes) while still allowing proton diffusion.84 Using wild-type S. ovata to directly consume the generated electrons, they were able to successfully produce acetate at high titers and efficiencies and demonstrated the feasibility of both reactor designs for future electrofuel applications.
Fluctuations in supply of renewable electricity, however, may be inevitable because of (economic) boundary conditions imposed by the grid. Fortunately, cyanobacteria can easily accommodate this, because they operate as ideal ‘rectifiers’ in relation to on/off rates of photon supply.64 In this respect they outperform organisms that catalyze electrosynthesis. In the latter systems, variations in rates of electron supply often lead to reaction-reversal and inactivation of the underlying biological processes and/or electrode surfaces.85,86
The feasibility of operating so-called on-site solar farms has often been both discussed and negated. Also Ooms et al.14 recently concluded that cyanobacterial biomass produced in such way will cost 14 $ per kg DW, which is multiple-fold above market price for energy applications. We note, however, that their estimate is pessimistic on several accounts for direct conversion by cyanobacteria (compare also Table 4): (i) the efficiency of (far) red LEDs can be as high as 85%,87 (ii) maintenance energy requirements in cyanobacteria are minimal,41 and (iii) the costs of renewable electricity may soon be 5-fold lower than assumed.14
| Process | PVCs | Photosynthesis | LEDs | Electrolyzer | Cupriavidus | Geobacter | η tot (%) | Strength | Weakness |
|---|---|---|---|---|---|---|---|---|---|
| Approximate efficiency estimates have been made of the subsequent steps in the S2P pathways discussed in this review. Their main strengths and weaknesses are also indicated. For further explanation: see text.a From: Giddings et al. 2015.84b From: Schuurmans et al. 2015.89 | |||||||||
| Solar fuel | 10 | 10 | Single step | Exciton annihilation | |||||
| LED fuel | 25 | 20b | 80 | 04 | Rectifier function | Exciton annihilation | |||
| Electrofuel | 25 | 80 | 80 | 16 | Efficiency | Volumetric prod. | |||
| Electrodirectfuel | 25 | 40a | 10 | Single step | Reactor design | ||||
The analysis of production costs can be extended to secreted products; using a kW h cost of $0.02, an electricity-to-LED conversion efficiency of 80%, the efficiency of 3.58%, and an energy content of ethylene of 4.94 × 107 J kg−1, the electricity costs of ethylene production with engineered cyanobacteria via LED illumination is around $15 per kg. This cost is still more than one order of magnitude greater than the current global ethylene price index ($1.06 per kg; May 2017). For ethylene, such a cost is clearly incompatible with commercial application; for products with much higher added-value (like fragrances or flavor compounds), however, the economics would improve. As an alternative, further work can be done to increase %CP towards ethylene.
In both electrofuel- and photosynthesis-based production processes, product titers have generally been significantly lower than in classical fermentations. This disadvantage, however, is offset in many cases by a much simpler chemical composition of the spent medium from which these products have to be recovered. This then also allows a much cheaper downstream processing, often via a more intense use of membrane filtration and decreased need for purification. For ethanol production by recombinant cyanobacteria, it has been calculated in the DEMA consortium, based upon innovations in the recovery of low concentrations of ethanol, that product titers as low as 1% (v/v) ethanol may already be sufficient for economic viability of ethanol recovery, when benchmarked against state of the practice ethanol distillation techniques used within 1st generation sugar cane ethanol production systems (C. Sheahan, University of Limerick (IRL), personal communication). Another important aspect is that many reactors for solar- and electro-fuel production will be mixed via gas-flow (that also supplies the required CO2). This opens the possibility to further simplify downstream processing via stripping of a volatile product from the reactor. Ethylene is an important example of such a product, but several other compounds also qualify, like ethanol, acetaldehyde, butanol, and butyraldehyde.
Conclusions
Ultimately, economic success of the approaches described in this review will be dictated by: (i) feasibility of upscaling; (ii) cost of reactor building and operation and of downstream processing, and (iii) product value. Where the feasibility of upscaling depends to a large degree on the challenges outlined, the costs of reactor building and operation depend significantly on the main production metrics in terms of rates and efficiencies, as analyzed in this review in several ways. Production rates describe volumetric productivity, the highest published of which were the production of isobutyraldehyde and ethanol by S. elongatus PCC7942 and Synechocystis sp. PCC6803, respectively. This volumetric productivity refers to the rate at which a product can be made in a reactor of a given size. Since fermenters and photo-bioreactors represent a significant fraction of the capital investment needed for a production facility, volumetric productivity has a significant impact on the required size and cost.Solar-to-product efficiency, in which the biggest strides were made in the recent electrofuel study by Liu et al.,9 represents the yield of product per amount of substrate, i.e. light energy. Solar energy is abundant and freely available on many locations on the planet, and as such it could be argued that yield is not as important a metric as it is for traditional fermentation approaches. But solar radiation is also rather dilute, which implies that photosynthesis-based approaches depend critically on the two-dimensional area of photon capture, either in the form of solar panels, light-sensitive electrodes, or the surface area of a cyanobacterial culture suspended in liquid. As such, higher efficiencies allow for smaller surface area requirements and are crucial for keeping costs down. In this sense, the maximum theoretical efficiencies calculated for the partial reactions of each of the approaches considered (Table 4) indicate that the combination of renewable electricity, water-splitting, and production via C. necator may ultimately yield the highest possible conversion efficiency, independent of latitude.14 But many additional factors impact the overall production metrics, including the rate of product formation, which may be inherently lower for the electrofuel approach, e.g. because of low rates of hydrogen transfer from the gas to the liquid phase.88
The assumed losses of solar energy at light capture by the PV cells (∼80%) and conversion of electricity to monochromatic light at the LEDs (∼50%), which jointly make a total loss of about 90%, do not outweigh the approximately 2-fold gain in S2P efficiency via the use of monochromatic far-red light. Nevertheless, both efficiency estimates are conservative: in the near future PV and LED efficiency may well surpass 25%39 and 80%,87 respectively, which would bring the areal efficiency of the LED-based approach (i.e.Fig. 1B) at least on par with regular agriculture (without the need for fertile soil for the former).
A further increase of PV cell efficiency to its current maximum90 is needed to bring the two approaches using cyanobacteria to the same maximal theoretical conversion efficiency (compare Table 4). These calculations do not account for the potential benefits of using a 3-D reactor over photo-bioreactors that have to maximize surface area for capturing sunlight (such as cost per kg of product, minimization of infection risks, controllability of pH, pCO2, temperature mixing, etc.). Table 4 also shows that the key step in all approaches is the efficiency of the light-harvesting step. The quantitative conversion of electronically excited states, resulting from solar radiation, into stable chemical-bond energy is a challenge for both inorganic and biological systems. Beyond this, it remains to be decided whether or not, the 2-step approaches for fuel production (i.e.Fig. 1A and B) will constitute an efficiency and/or cost improvement over the direct solar conversion approach (Fig. 1C).
For all three approaches depicted in Fig. 1, further progress in genetically engineering the microbial catalyst using the rapidly developing synthetic biology toolbox will allow further increases in %CP, and thereby, also in overall efficiency. For selected products, such as ethanol, lactic acid and 2,3-butane-diol, the carbon partitioning has surpassed the 50% threshold in small-scale laboratory experiments.13 However, this high %CP comes with a fitness cost for the engineered strains as it drains significant resources from anabolic processes. This competition between product and biomass formation, has LED to the appearance of mutant strains that lost the ability to form product,65,68,91,92 particularly under conditions of unrestricted growth. More recently, Du et al. have shown that this apparent genetic instability of production strains comes from the fitness burden derived from deviating carbon itself away from biomass, and towards product.93 This was done by allosterically modulating the flux towards lactic acid without changing the levels of the heterologously expressed lactate dehydrogenase of Lactococcus lactis. The authors observed that the rate of appearance of non-producing mutants increased with the %CP. In electrofuel production systems, genetic instability remains to be reported. However, similar to what happens in many other biotechnological systems,94 whenever microbial fitness is not aligned with product formation, Darwinian selection is likely to favor faster-growing, non-producing, strains, particularly during industrial-scale cultivations.95 So, it is highly advisable that future work in this field will deploy strategies to minimize this hurdle, such that the large-scale application can be rendered feasible.
Several solutions have been proposed to minimize the fitness burden of product formation. For instance, the development of inducible systems responsive to environmental cues that can uncouple the expression of a production pathway from growth has been thought after in cyanobacteria.96,97 These genetic tools can still be further improved, particularly for the species used in the electrofuel approach (e.g. C. necator), where the engineering of such synthetic regulatory circuits still present a significant challenge.98 As an alternative, growth can be limited by lack of a specific nutrient, which then – if the proper nutrient is selected – may lead to ‘overflow metabolism’ as long as sufficient energy (in the form of light) is available. Another solution that has been proposed to better align cellular fitness with product formation involves the implementation of growth-coupled strategies.99 Traditionally, relying on systems biology approaches, the model-based design of such strategies would couple the energy or co-factor regeneration ability of cells to product formation.100–102 This approach has been discussed extensively and tested in silico for cyanobacteria,103–107 but never experimentally. Very recently, we have developed an alternative strategy that also achieves growth-coupled production.108 This latter approach involves the deletion of the genes encoding the native reactions that cells have to recycle side-products produced in anabolic pathways. This approach has been experimentally tested in Synechocystis PCC6803, resulting in the first photoautotrophic grow-coupled cell factories. While not applicable to every product of choice, a computational tool (FRUITS) has been made available that allows one to ‘Find Reactions Usable In Tapping Side-products’ (http://https://gitlab.com/mmp-uva/fruits). This is applicable to any production system for which a genome-scale model is available; including some popular amongst the electrofuel production systems such as the modeled C. necator.109 It is interesting to note that while growth-coupled production systems have not yet rationally been designed for electrofuel hosts, some of the organisms used there (e.g. acetogenic bacteria9) have naturally evolved this trait. In those cases, no stability issues are to be expected for those very specific products.
Beyond earth-based applications, both cyanobacteria-based systems and electrofuel setups also have the potential to contribute to manned space missions. Launching payloads into space is extremely expensive, and technologies that utilize local resources such as solar energy and CO2 for the manufacturing of fuels, chemicals, and pharmaceuticals, as well as for other applications, could significantly reduce costs and improve mission feasibility and safety.110 Although cyanobacteria and C. necator (in the context of traditional fermentation) have been actively explored for such purposes,111 the deployment of electrofuel systems in this context is novel, and at least as promising.
Altogether, further work to systematically characterize S2P efficiency, production rate, and long-term stability of production strains is needed for a wider range of electrofuel and cyanobacteria-based setups. These experiments need to be conducted more rigorously and consistently than the published proof-of-principle studies that mainly used flasks, bottles and/or column bioreactors with ambiguously described illumination setups, which make it difficult to accurately determine the relevant surface area for efficiency calculations. Ideally, such studies will utilize flat-panel photobioreactors with clearly defined surface areas and tested with illumination setups using both (artificial) sunlight and optimized monochromatic light.
To bridge the considerable gap between costs and benefit of solar-driven conversions of CO2 to product, catalyzed by living microorganisms, significant further research and funding to achieve scale-up success will be required. This holds true in particular for the electrofuel and LED-powered cyanobacteria approaches, which, although promising, are currently still at the proof-of-principle stage. Although the road to commercialization will require perseverance, targeted action at upscaling, and rigorous analysis of production metrics, the future impact of solar-driven CO2-fixation to produce valuables justifies the effort and can no longer be ignored.
Conflicts of interest
Klaas J. Hellingwerf is the scientific advisor of Photanol B.V., a University of Amsterdam spin-off company aiming at commercializing sustainable applications with cyanobacteria. He and the other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.Acknowledgements
Klaas J. Hellingwerf was supported by the Dutch Ministry of Economic Affairs, Agriculture, and Innovation (research program Bio-Solar Cells) and the FP7 DEMA project, grant agreement no. 309086. The Netherlands Organization for Scientific Research (NWO) supported Filipe Branco dos Santos through Solar-2-product grant 733 000 005.References
- N. Nakicenovic and R. Swart, IPCC Special Report on Emissions Scenarios: A special report of Working Group III of the Intergovernmental Panel on Climate Change, 2000.
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- J. Barber, Chem. Soc. Rev., 2009, 38, 185–196 RSC.
- D. Gust, T. A. Moore and A. L. Moore, Faraday Discuss., 2012, 155, 9–26 RSC.
- K. D. Yang, C. W. Lee, K. Jin, S. W. Im and K. T. Nam, J. Phys. Chem. Lett., 2017, 8, 538–545 CrossRef CAS PubMed.
- S. Gao, Y. Lin, X. Jiao, Y. Sun, Q. Luo, W. Zhang, D. Li, J. Yang and Y. Xie, Nature, 2016, 529, 68–71 CrossRef CAS PubMed.
- Y. H. Choi, Y. J. Jang, H. Park, W. Y. Kim, Y. H. Lee, S. H. Choi and J. S. Lee, Appl. Catal., B, 2017, 202, 605–610 CrossRef CAS.
- P. Vandamme and T. Coenye, Int. J. Syst. Evol. Microbiol., 2004, 54, 2285–2289 CrossRef PubMed.
- C. Liu, B. C. Colón, M. Ziesack, P. A. Silver and D. G. Nocera, Science, 2016, 352, 1210–1213 CrossRef CAS PubMed.
- T. Zhang, Science, 2015, 350, 738–739 CrossRef CAS PubMed.
- D. R. Lovley and K. P. Nevin, Curr. Opin. Biotechnol., 2013, 24, 385–390 CrossRef CAS PubMed.
- K. J. Hellingwerf and M. J. Teixeira de Mattos, J. Biotechnol., 2009, 142, 87–90 CrossRef CAS PubMed.
- S. A. Angermayr, A. Gorchs Rovira and K. J. Hellingwerf, Trends Biotechnol., 2015, 33, 352–361 CrossRef CAS PubMed.
- M. D. Ooms, C. T. Dinh, E. H. Sargent and D. Sinton, Nat. Commun., 2016, 7, 12699 CrossRef CAS PubMed.
- A. Mohr and S. Raman, Energy Policy, 2013, 63, 114–122 CrossRef PubMed.
- S. N. Naik, V. V. Goud, P. K. Rout and A. K. Dalai, Renewable Sustainable Energy Rev., 2010, 14, 578–597 CrossRef CAS.
- M. L. Ghirardi, M. C. Posewitz, P.-C. Maness, A. Dubini, J. Yu and M. Seibert, Annu. Rev. Plant Biol., 2007, 58, 71–91 CrossRef CAS PubMed.
- C. Xiang, A. Z. Weber, S. Ardo, A. Berger, Y. K. Chen, R. Coridan, K. T. Fountaine, S. Haussener, S. Hu, R. Liu, N. S. Lewis, M. A. Modestino, M. M. Shaner, M. R. Singh, J. C. Stevens, K. Sun and K. Walczak, Angew. Chem., Int. Ed., 2016, 55, 12974–12988 CrossRef CAS PubMed.
- R. J. Conrado, C. A. Haynes, B. E. Haendler and E. J. Toone, in Advanced Biofuels and Bioproducts, ed. J. W. Lee, Springer, 2013, pp. 1037–1064 Search PubMed.
- H. Li, P. H. Opgenorth, D. G. Wernick, S. Rogers, T.-Y. Wu, W. Higashide, P. Malati, Y.-X. Huo, K. M. Cho and J. C. Liao, Science, 2012, 335, 1596 CrossRef CAS PubMed.
- D. G. Nocera, Acc. Chem. Res., 2012, 45, 767–776 CrossRef CAS PubMed.
- C. Liu, J. J. Gallagher, K. K. Sakimoto, E. M. Nichols, C. J. Chang, M. C. Y. Chang and P. Yang, Nano Lett., 2015, 15, 3634–3639 CrossRef CAS PubMed.
- E. M. Nichols, J. J. Gallagher, C. Liu, Y. Su, J. Resasco, Y. Yu, Y. Sun, P. Yang, M. C. Y. Chang and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 11461–11466 CrossRef CAS PubMed.
- S. Atsumi, W. Higashide and J. C. Liao, Nat. Biotechnol., 2009, 27, 1177–1180 CrossRef CAS PubMed.
- Z. Gao, H. Zhao, Z. Li, X. Tan and X. Lu, Energy Environ. Sci., 2012, 5, 9857–9865 CAS.
- J. W. K. Oliver, I. M. P. Machado, H. Yoneda and S. Atsumi, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 1249–1254 CrossRef CAS PubMed.
- A. M. Ruffing, Front. Bioeng. Biotechnol., 2014, 2, 17 Search PubMed.
- E. I. Lan, D. S. Chuang, C. R. Shen, A. M. Lee, S. Y. Ro and J. C. Liao, Metab. Eng., 2015, 31, 163–170 CrossRef CAS PubMed.
- Y. Hirokawa, Y. Maki, T. Tatsuke and T. Hanai, Metab. Eng., 2016, 34, 97–103 CrossRef CAS PubMed.
- T. Zavřel, H. Knoop, R. Steuer, P. R. Jones, J. Červený and M. Trtílek, Bioresour. Technol., 2016, 202, 142–151 CrossRef PubMed.
- J. P. Torella, C. J. Gagliardi, J. S. Chen, D. K. Bediako, B. Colón, J. C. Way, P. A. Silver and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 2337–2342 CrossRef CAS PubMed.
- M. De Deng and J. R. Coleman, Appl. Environ. Microbiol., 1999, 65, 523–528 CAS.
- Q. Chen, D. Montesarchio and K. J. Hellingwerf, Advances in Botanical Research, 2016, vol. 79, pp. 43–62 Search PubMed.
- B. Sialve, N. Bernet and O. Bernard, Biotechnol. Adv., 2009, 27, 409–416 CrossRef CAS PubMed.
- R. H. Wijffels, M. J. Barbosa and M. H. M. Eppink, Biofuels, Bioprod. Biorefinin., 2010, 4, 287–295 CrossRef CAS.
- E. Tyystjarvi, Coord. Chem. Rev., 2008, 252, 361–376 CrossRef CAS.
- J. Jung and H.-S. Kim, Photochem. Photobiol., 1990, 52, 1003–1009 CrossRef CAS.
- L. Nedbal, M. Trtílek, J. Červený, O. Komárek and H. B. Pakrasi, Biotechnol. Bioeng., 2008, 100, 902–910 CrossRef CAS PubMed.
- M. A. Green, Nat. Publ. Gr., 2016, 1, 1–4 Search PubMed.
- OSLON SSL80Datasheet Version 2.0, http://www.osram-os.com/Graphics/XPic4/00100986_0.pdf.
- W. Du, J. A. Jongbloets, H. Pineda Hernández, F. J. Bruggeman, K. J. Hellingwerf and F. Branco dos Santos, Algal Res., 2016, 20, 118–125 CrossRef.
- H. Niederholtmeyer, B. T. Wolfstädter, D. F. Savage, P. A. Silver and J. C. Way, Appl. Environ. Microbiol., 2010, 76, 3462–3466 CrossRef CAS PubMed.
- S. G. Hays, L. L. W. Yan, P. A. Silver and D. C. Ducat, J. Biol. Eng., 2017, 11, 4 CrossRef PubMed.
- N. Antonovsky, S. Gleizer, E. Noor, Y. Zohar, E. Herz, U. Barenholz, L. Zelcbuch, S. Amram, A. Wides, N. Tepper, D. Davidi, Y. Bar-On, T. Bareia, D. G. Wernick, I. Shani, S. Malitsky, G. Jona, A. Bar-Even and R. Milo, Cell, 2016, 166, 115–125 CrossRef CAS PubMed.
- J. Dexter, P. Armshaw, C. Sheahan and J. T. Pembroke, J. Appl. Microbiol., 2015, 119, 11–24 CrossRef CAS PubMed.
- Z. Zhu, G. Luan, X. Tan, H. Zhang and X. Lu, Biotechnol. Biofuels, 2017, 10, 93 CrossRef PubMed.
- Comparison of commonly used technologies for the cultivation of algae, 2016.
- J. H. de Vree, R. Bosma, M. Janssen, M. J. Barbosa and R. H. Wijffels, Biotechnol. Biofuels, 2015, 8, 215 CrossRef PubMed.
- J. Guan, S. A. Berlinger, X. Li, Z. Chao, V. Sousa e Silva, S. Banta and A. C. West, J. Biotechnol., 2017, 245, 21–27 CrossRef PubMed.
- L. Mai, M. Yan and Y. Zhao, Nature, 2017, 546, 469–470 CrossRef CAS PubMed.
- K. T. Fountaine, H. J. Lewerenz and H. A. Atwater, Nat. Commun., 2016, 7, 13706 CrossRef CAS PubMed.
- J. Müller, D. MacEachran, H. Burd, N. Sathitsuksanoh, C. Bi, Y. C. Yeh, T. S. Lee, N. J. Hillson, S. R. Chhabra, S. W. Singer and H. R. Beller, Appl. Environ. Microbiol., 2013, 79, 4433–4439 CrossRef PubMed.
- E. Grousseau, J. Lu, N. Gorret, S. E. Guillouet and A. J. Sinskey, Appl. Microbiol. Biotechnol., 2014, 98, 4277–4290 CrossRef CAS PubMed.
- T. Oda, K. Oda, H. Yamamoto, A. Matsuyama, M. Ishii, Y. Igarashi and H. Nishihara, Microb. Cell Fact., 2013, 12, 2 CrossRef CAS PubMed.
- I. Voss and A. Steinbüchel, Metab. Eng., 2006, 8, 66–78 CrossRef CAS PubMed.
- C. Ewering, F. Heuser, J. K. Benölken, C. O. Brämer and A. Steinbüchel, Metab. Eng., 2006, 8, 587–602 CrossRef CAS PubMed.
- J. Overhage, A. Steinbüchel and H. Priefert, Appl. Environ. Microbiol., 2002, 68, 4315–4321 CrossRef CAS PubMed.
- B. Y. Jeon, J. Y. Yi, I. L. Jung and D. H. Park, Adv. Microbiol., 2013, 3, 42–45 CrossRef CAS.
- M. Köpke, C. Held, S. Hujer, H. Liesegang, A. Wiezer, A. Wollherr, A. Ehrenreich, W. Liebl, G. Gottschalk and P. Dürre, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 13087–13092 CrossRef PubMed.
- M. Kopke, C. Mihalcea, F. Liew, J. H. Tizard, M. S. Ali, J. J. Conolly, B. Al-Sinawi and S. D. Simpson, Appl. Environ. Microbiol., 2011, 77, 5467–5475 CrossRef CAS PubMed.
- B. Schiel-Bengelsdorf and P. Dürre, FEBS Lett., 2012, 586, 2191–2198 CrossRef CAS PubMed.
- T. Ueki, K. P. Nevin, T. L. Woodard and D. R. Lovley, MBio, 2014, 5, 19–23 CrossRef PubMed.
- D. C. Ducat, J. A. Avelar-Rivas, J. C. Way and P. A. Silvera, Appl. Environ. Microbiol., 2012, 78, 2660–2668 CrossRef CAS PubMed.
- P. E. Savakis, S. A. Angermayr and K. J. Hellingwerf, Metab. Eng., 2013, 20C, 121–130 CrossRef PubMed.
- S. A. Angermayr, M. Paszota and K. J. Hellingwerf, Appl. Environ. Microbiol., 2012, 78, 7098–7106 CrossRef CAS PubMed.
- A. M. Varman, Y. Yu, L. You and Y. J. Tang, Microb. Cell Fact., 2013, 12, 117 CrossRef PubMed.
- P. Savakis, X. Tan, W. Du, F. Branco Dos Santos, X. Lu and K. J. Hellingwerf, J. Biotechnol., 2015, 195, 46–51 CrossRef CAS PubMed.
- J. H. Jacobsen and N. U. Frigaard, Metab. Eng., 2014, 21, 60–70 CrossRef CAS PubMed.
- B. Wang, S. Pugh, D. R. Nielsen, W. Zhang and D. R. Meldrum, Metab. Eng., 2013, 16, 68–77 CrossRef CAS PubMed.
- E. I. Lan, S. Y. Ro and J. C. Liao, Energy Environ. Sci., 2013, 6, 2672 CAS.
- C. R. Shen and J. C. Liao, Energy Environ. Sci., 2012, 5, 9574–9583 CAS.
- H. Li and J. C. Liao, Microb. Cell Fact., 2013, 14 DOI:10.1186/1475-2859-12-4.
- J. Zhou, H. Zhang, Y. Zhang, Y. Li and Y. Ma, Metab. Eng., 2012, 14, 394–400 CrossRef CAS PubMed.
- T. Kusakabe, T. Tatsuke, K. Tsuruno, Y. Hirokawa, S. Atsumi, J. C. Liao and T. Hanai, Metab. Eng., 2013, 20C, 101–108 CrossRef PubMed.
- M. Sakai, T. Ogawa, M. Matsuoka and H. Fukuda, J. Ferment. Bioeng., 1997, 84, 434–443 CrossRef CAS.
- F. K. Davies, V. H. Work, A. S. Beliaev and M. C. Posewitz, Front. Bioeng. Biotechnol., 2014, 2, 21 Search PubMed.
- E. Englund, B. Pattanaik, S. J. K. Ubhayasekera, K. Stensjö, J. Bergquist and P. Lindberg, PLoS One, 2014, 9, 1–8 Search PubMed.
- C. Formighieri and A. Melis, Planta, 2014, 240, 309–324 CrossRef CAS PubMed.
- F. K. Bentley, A. Zurbriggen and A. Melis, Mol. Plant, 2014, 7, 71–86 CrossRef CAS PubMed.
- C. Halfmann, L. Gu, W. Gibbons and R. Zhou, Appl. Microbiol. Biotechnol., 2014, 98, 9869–9877 CrossRef CAS PubMed.
- T. Yoshino, Y. Liang, D. Arai, Y. Maeda, T. Honda, M. Muto, N. Kakunaka and T. Tanaka, Appl. Microbiol. Biotechnol., 2015, 99, 1521–1529 CrossRef CAS PubMed.
- X. Tan, W. Du and X. Lu, Appl. Microbiol. Biotechnol., 2015, 99, 2147–2154 CrossRef CAS PubMed.
- Y. Xue, Y. Zhang, S. Grace and Q. He, J. Appl. Phycol., 2014, 26, 219–226 CrossRef CAS.
- C. G. S. Giddings, K. P. Nevin, T. Woodward, D. R. Lovley and C. S. Butler, Front. Microbiol., 2015, 6, 468 Search PubMed.
- S. J. Andersen, I. Pikaar, S. Freguia, B. C. Lovell, K. Rabaey and R. A. Rozendal, Environ. Sci. Technol., 2013, 47, 5488–5494 CrossRef CAS PubMed.
- K. Rabaey and R. A. Rozendal, Nat. Rev. Microbiol., 2010, 8, 706–716 CrossRef CAS PubMed.
- J. Zhong, D. Chen, H. Xu, W. Zhao, J. Sun and Z. Ji, J. Alloys Compd., 2017, 695, 311–318 CrossRef CAS.
- M. Götz, J. Lefebvre, F. Mörs, A. McDaniel Koch, F. Graf, S. Bajohr, R. Reimert and T. Kolb, Renew. Energy, 2016, 85, 1371–1390 CrossRef.
- R. M. Schuurmans, P. Van Alphen, J. M. Schuurmans, H. C. P. Matthijs and K. J. Hellingwerf, PLoS One, 2015, 10, 1–17 Search PubMed.
- F. Dimroth, M. Grave, P. Beutel, U. Fiedeler, C. Karcher, T. N. D. Tibbits, E. Oliva, G. Siefer, M. Schachtner, A. Wekkeli, A. W. Bett, R. Krause, M. Piccin, N. Blanc, C. Drazek, E. Guiot, B. Ghyselen, T. Salvetat, A. Tauzin, T. Signamarcheix, A. Dobrich, T. Hannappel and K. Schwarzburg, Prog. Photovoltaics, 2014, 22, 277–282 CAS.
- J. Ungerer, L. Tao, M. Davis, M. Ghirardi, P.-C. Maness and J. Yu, Energy Environ. Sci., 2012, 5, 8998 CAS.
- T. Kusakabe, T. Tatsuke, K. Tsuruno, Y. Hirokawa, S. Atsumi, J. C. Liao and T. Hanai, Metab. Eng., 2013, 20, 101–108 CrossRef CAS PubMed.
- W. Du, S. A. Angermayr, J. A. Jongbloets, D. Molenaar, H. Bachmann, K. J. Hellingwerf and F. Branco dos Santos, ACS Synth. Biol., 2017, 6, 395–401 CrossRef CAS PubMed.
- E. Darmon and D. R. Leach, Microbiol. Mol. Biol. Rev., 2014, 78, 1–39 CrossRef CAS PubMed.
- H. Bachmann, D. Molenaar, F. Branco Dos Santos and B. Teusink, FEMS Microbiol. Rev., 2017, 41, S201–S219 CrossRef PubMed.
- F. Branco dos Santos, W. Du and K. J. Hellingwerf, Front. Bioeng. Biotechnol., 2014, 2 DOI:10.3389/fbioe.2014.00036.
- B. M. Berla, R. Saha, C. M. Immethun, C. D. Maranas, T. S. Moon and H. B. Pakrasi, Front. Microbiol., 2013, 4, 246 Search PubMed.
- S. Gruber, D. Schwendenwein, Z. Magomedova, E. Thaler, J. Hagen, H. Schwab and P. Heidinger, J. Biotechnol., 2016, 235, 92–99 CrossRef CAS PubMed.
- A. M. Feist, D. C. Zielinski, J. D. Orth, J. Schellenberger, M. J. Herrgard and B. O. Palsson, Metab. Eng., 2010, 12, 173–186 CrossRef CAS PubMed.
- C. R. Shen, E. I. Lan, Y. Dekishima, A. Baez, K. M. Cho and J. C. Liao, Appl. Environ. Microbiol., 2011, 77, 2905–2915 CrossRef CAS PubMed.
- K. M. Smith and J. C. Liao, Metab. Eng., 2011, 13, 674–681 CrossRef CAS PubMed.
- S. S. Fong, A. P. Burgard, C. D. Herring, E. M. Knight, F. R. Blattner, C. D. Maranas and B. O. Palsson, Biotechnol. Bioeng., 2005, 91, 643–648 CrossRef CAS PubMed.
- S. Gudmundsson and J. Nogales, Mol. Biosyst., 2015, 11, 60–70 RSC.
- H. Knoop and R. Steuer, Front. Bioeng. Biotechnol., 2015, 3 DOI:10.3389/fbioe.2015.00047.
- J. Nogales, S. Gudmundsson and I. Thiele, Bioengineered, 2013, 4, 158–163 CrossRef PubMed.
- K. Shabestary and E. P. Hudson, Metab. Eng. Commun., 2016, 3, 216–226 CrossRef.
- P. Erdrich, H. Knoop, R. Steuer and S. Klamt, Microb. Cell Fact., 2014, 13, 128 CrossRef PubMed.
- W. Du, J. A. Jongbloets, C. van Boxtel, H. Pineda Hernández, D. Lips, B. G. Olivier, K. J. Hellingwerf and F. Branco dos Santos, Under review.
- J. Park, T. Kim and S. Lee, BMC Syst. Biol., 2011, 5, 101 CrossRef CAS PubMed.
- A. A. Menezes, M. G. Montague, J. Cumbers, J. A. Hogan and A. P. Arkin, J. R. Soc., Interface, 2015, 12, 20150803 CrossRef PubMed.
- A. A. Menezes, J. Cumbers, J. A. Hogan and A. P. Arkin, J. R. Soc., Interface, 2015, 12, 20140715 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: File with supplementary data including the description of the calculations made during this analysis. See DOI: 10.1039/c7ee02212c |
| ‡ These authors share first authorship. |
| § These authors share last authorship. |
| This journal is © The Royal Society of Chemistry 2018 |