Comment on “Alternative strategy for a safe rechargeable battery” by M. H. Braga, N. S. Grundish, A. J. Murchison and J. B. Goodenough, Energy Environ. Sci., 2017, 10, 331–336
Daniel A.
Steingart
 *a and
Venkatasubramanian
Viswanathan
*a and
Venkatasubramanian
Viswanathan
 b
b
aDepartment of MAE/ACEE, Princeton University, Princeton, NJ, USA. E-mail: steingart@princeton.edu
bDepartment of ME, Carnegie Mellon University, Pittsburgh, PA, USA
First published on 5th December 2017
Braga et al.1 demonstrated a series of electrochemical cells consisting of: (a) alkali metal anodes; (b) a barium anti-perovskite separator; and (c) a variety of cathodes. The work shows that certain cells produce far more energy than standard faradaic analysis of the mass of the active oxidizing agent would allow, for e.g. the lithium anode/sulfur cathode combination, shown in Fig. 1 of the paper. Our comment is focused on the cells which exhibit this “overcapacity.”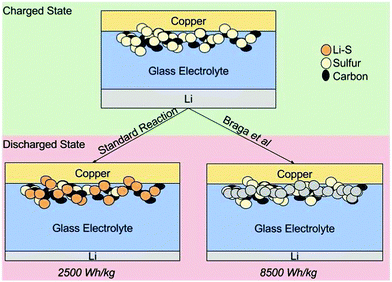 | ||
| Fig. 1 Schematic representation of the discharged state of a standard Li–S battery (left) and of the metal plating scheme (right) proposed by Braga et al. | ||
The overcapacity of the cell is not attributed to an unintended oxidizing agent, and the proposed mechanism involves neither the anode nor cathode undergoing any net redox in the overall reaction. Thus, the effective state of the positive electrode after “discharge” is that of unreduced cathode material and effectively unoxidized electrodeposited alkali metal.
In this comment we show that no mechanistic description is sufficient to connect the beginning and ending states while energy is released. The mechanistic process as described in the paper constitutes a first-law violation.
According to the mechanism proposed by Braga et al., schematically shown in Fig. 4, for the Li–S cell, lithium metal plates during discharge at the cathode:
| Li → Li+ + e− at negative electrode | (1) |
| Li+ + e− → Li at positive; ΔGnet = 0 | (2) |
Following the mechanism proposed by Braga et al., the sulfur does not change its chemical state
| S → S; ΔGnet = 0 | (3) |
| Cu → Cu, which has ΔGnet = 0 | (4) |
Braga et al. argue that there is a difference in the Fermi levels of the current collectors determined by the difference in work functions of the two current collectors, EF,A = 3.05 VSHE and EF,A = 0.40 VSHE. Once the cell begins discharging, the Fermi level at both ends of the current collector is pinned by the redox processes occurring at the anode and cathode, respectively.
It is worth highlighting that even if the sulfur “redox center” acts a redox mediator for the same eventual states, for example through an intermediate state, Li2S, according to the reactions,
| 2Li → 2Li+ + 2e− at anode |
| 2Li+ + 2e− + S → Li2S. |
For each cell in the work of Braga et al. an open circuit potential of that between the anode and the cathode is initially established. However, taken as a state machine, the cell cannot produce energy by simply moving the chemical species across the separator without a net reaction.
Thus, while the experimental result of Braga et al. is compelling as a solid state electrolyte with a lithium and sodium electrolyte capable of surviving many cycles, the metal redeposition mechanism described by Braga et al., cannot result in an electrochemical cell which produces energy.
Conflicts of interest
There are no conflicts to declare.References
- M. H. Braga, N. S. Grundish, A. J. Murchison and J. B. Goodenough, Energy Environ. Sci., 2017, 10, 331–336 CAS
.
- D. M. Kolb, M. Przasnyski and H. Gerischer, J. Electroanal. Chem. Interfacial Electrochem., 1974, 54, 25–38 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2018 |
