Two luminescent lanthanide(iii) metal–organic frameworks as chemosensors for high-efficiency recognition of Cr(vi) anions in aqueous solution†
Abstract
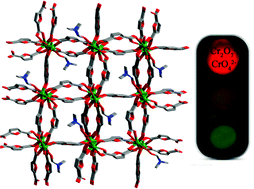
Two new lanthanide(III) metal–organic frameworks (MOFs) {[(CH3)2NH2]2[Ln4(FDA)7(DMF)2]·0.5DMF}n [Ln = Eu (1), and Tb (2)] based on furan-2,5-dicarboxylic acid (H2FDA) have been successfully assembled and well characterized in detail. These MOFs are isostructural and demonstrate 12-connected sqc15 topologies, which are rarely observed in MOF chemistry, especially in lanthanide(III) MOFs. Moreover, these two MOFs could show a tolerance towards moisture and organic solvents and satisfactory chemical stabilities. More importantly, they exhibit sensitive and selective luminescence quenching response towards Cr2O72− and CrO42− anions in aqueous solution with the average quenching Ksv values of 1.25 × 104 L mol−1 (Cr2O72−) and 3.56 × 103 L mol−1 (CrO42−) for 1 and 1.46 × 104 L mol−1 (Cr2O72−) and 4.35 × 103 L mol−1 (CrO42−) for 2 and the detection limits of 1.14 × 10−4 mol L−1 (Cr2O72−) and 1.12 × 10−4 mol L−1 (CrO42−) for 1 and 7.42 × 10−5 mol L−1 (Cr2O72−) and 1.27 × 10−4 mol L−1 (CrO42−) for 2. The high quenching Ksv values and low detection limits make them more feasible in sensing Cr(VI) anions in aqueous solution. The possible detection mechanism has been discussed in detail.
- This article is part of the themed collection: Spotlight Collection: MOF Sensors


 Please wait while we load your content...
Please wait while we load your content...