Open Access Article
Open Access ArticleProtonation and water exchange kinetics in sandwich polyoxometalates†
C. André
Ohlin
 * and
Magda
Pascual-Borràs
* and
Magda
Pascual-Borràs
Department of Chemistry, Umeå University, Umeå, Sweden. E-mail: andre.ohlin@umu.se; Web: http://moleculargeo.chem.umu.se
First published on 5th September 2018
Abstract
Density functional theory is used to explore the locus and consequences of protonation in [Zn4(HO)2(PW9O34)2]10−. The results are used to explain recent observations regarding the contrasting pH effects on the water-ligand exchange in [Mn4(H2O)2(P2W15O56)2]16− and [Co4(H2O)2(P2W15O56)2]16−, and the general effect of protonation on solvent exchange in metal oxides is discussed.
Introduction
Polyoxometalates are discrete metal oxide clusters of V, Nb, Ta, Mo and W in their respective highest oxidation states, and exhibit a large structural diversity.1,2 This diversity is mirrored by the broad range of chemistries that this class of molecules demonstrates. In addition to being attractive as oxidative and reductive catalysts due to the absence of easily degradable organic moieties, polyoxometalates make interesting models for the study of geochemically relevant reactions, as many polyoxometalates are nanometre-sized and resemble mineral fragments and surface features in terms of size and functional groups.3–5Polyoxometalates can also be used as ligands to immobilise mineral-like fragments of other metal oxide classes. The Wells–Dawson polyoxometalates [M4(H2O)2(PW9O34)2]10− (1) and [M4(H2O)2(P2W15O56)2]16− (2) – where M is Mn(II),6 Fe(III),7 Co(II),8,9 Ni(II),6 Cu(II) or8,9 Zn(II),8,9– contain a birnessite-like sheet of M–O units sandwiched between two polyoxometalate ligands, and with aquo-ligands bound to the outer M sites. This arrangement offers a rare opportunity to probe the chemistry of small ligands, such as water, bound to mineral-like surfaces using solution phase methods. In particular, there is a limited amount of data on ligand exchange on metal oxide centres surrounded only by other metal oxide centres, where the system is a discrete cluster with a well-defined structure.
Reports that [Co4(H2O)2(PW9O34)2]10− was capable of catalysing the oxidation of water10 stimulated an interest in the interaction of water with this type of ion, leading to the simultaneous study by two groups of the water-exchange kinetics of [Co4(H2O)2(P2W15O56)2]16−,11 and [Co4(H2O)2(PW9O34)2]10−,11,12 using Swift–Connick formalism.13,14 While the systems exhibit a two-proton protonation event with a pKa of ca. 4.2,11 the rates of exchange were found to be pH-independent. The location of protonation was suggested to be on the polyoxometalate ligand, in particular as the pKa of the aquo-ligand is likely to be considerably higher than the determined pKa. This is, however, in contrast to earlier work, where the locus of protonation was assigned to the aquo ligand,15 but consistent with assignments based on molecular electrostatic potentials.16
Sharma et al. determined the rates of aquo-ligand exchange on the structurally similar [Mn4(H2O)2(P2W15O56)2]16− ion, also using Swift–Connick formalism.17 In contrast with the behaviour of the Co(II)-derivative, the rate of ligand exchange on the Mn(II)-derivative shows profound dependence on the pH. Here, the rate of exchange increases by a factor of 15 as the pH is lowered from 6.0 to 3.5. Yet, this molecule too exhibits a two-proton protonation event with a pKa of ca. 4.4, which is very similar to the determined pKa of the Co(II) cluster, and is likely to be associated with the same protonation locus.
While these Wells–Dawson molecules are of interest in themselves, the issue of protonation of metal oxide materials – both as discrete clusters and extended mineral and catalyst surfaces – is of much broader importance. Being able to predict loci of protonation and the effect of protonation is thus of considerable interest. In addition, the surface charge density of [M4(H2O)2(PW9O34)2]10− is ca −0.2 C m−2, which is comparable with that of common minerals, and makes these polyoxometalate clusters particularly interesting targets.
Here we attempt to computationally determine the loci of protonation in Wells–Dawson complexes, explain the difference in pH dependence of rates of aquo-ligand exchange between the Co(II) and Mn(II) derivatives, and tie these conclusions to more general observations of aquo-ligand exchanges in metal oxide materials.
Results and discussion
Computations using density functional theory (DFT) at the PBE018/def2-svp19 and PBE0/def2-tzvp levels of theory together with implicit solvation through the polarizable continuum model20 as implemented in G1621 were focused on the zinc(II)-containing molecule [Zn4(H2O)2(PW9O34)2]10− over the paramagnetic target molecules [Mn4(H2O)2(P2W15O56)2]16−, [Co4(H2O)2(P2W15O56)2]16− and [Co4(H2O)2(PW9O34)2]10− owing to the difficulty in accurate modelling paramagnetic systems by DFT, and the smaller size of the [PW9O34]9− ligand, while still having essentially the same structure in the proximity of the M4 plane. Unless otherwise specified, results reported in the text were obtained at PBE0/def2-tzvp with PCM to approximate solvation in water. Partial charges were computed as natural atomic charges from Natural Bond Order analysis,22 or using the Mesler–Singh–Kollman scheme using UFF radii,23 or Breneman's modified CHelp scheme using radii of 1.39 and 1.80 Å for Zn and W,24 respectively, or the Hu, Lu and Yan charge-fitting method.25 Additional tabulated energies, optimised structures etc. are provided in the ESI.†Exploration of protonation loci thus focused on [Zn4(H2O)2(PW9O34)2]10− due to its small size and diamagnetic nature. Protonation of all oxygen sites was explored (see Fig. 1) and each site was protonated symmetrically by two protons to reproduce the experimental observation of protonation in these molecules being a two-proton protonation event, and the corresponding structure was optimised. The relative energies and Zn–OH2 bond distances for the protonated molecules, and the partial charges for the corresponding oxygen sites in the unprotonated molecule, are tabulated in Table 1.
| Entry | Site | Δε (kcal mol−1) | d(M–OH2) (Å) | NBOa (a.u.) | MKUFFb (a.u.) | CHelpGc (a.u.) | HLYGAtd (a.u.) |
|---|---|---|---|---|---|---|---|
| Geometries optimised at pbe0/def2-tzvp with PCM (water). The d(M–OH2) in the non-protonated form is 2.236 Å. Partial charges were calculated for the unprotonated molecule at pbe0/def2-tzvp.a Natural atomic charges from Natural Bond Order analysis.22b The Mesler–Singh–Kollman scheme, using UFF radii.23c Breneman's modified CHelp scheme, using radii of 1.39 and 1.80 Å for Zn and W, respectively.24d The Hu, Lu, and Yang charge fitting method using G09 standard atom densities.25 | |||||||
| 1 | A (μ2) | 22.038 | 2.207 | −0.8962 | −0.8249 | −0.8623 | −0.7099 |
| 2 | B (μ2) | 20.798 | 2.199 | −0.9154 | −0.8872 | −0.8608 | −0.7214 |
| 3 | C (μ2) | 19.601 | 2.199 | −0.9041 | −0.8214 | −0.8384 | −0.7307 |
| 4 | D (μ2) | 17.639 | 2.202 | −0.9121 | −0.8391 | −0.8253 | −0.7342 |
| 5 | E (μ2) | 16.933 | 2.200 | −0.9145 | −0.8684 | −0.8235 | −0.7276 |
| 6 | F (μ2) | 16.457 | 2.193 | −0.9119 | −0.8654 | −0.8414 | −0.7254 |
| 7 | G (μ2) | 18.579 | 2.199 | −0.9117 | −0.9102 | −0.8475 | −0.7810 |
| 8 | H (μ2) | 16.735 | 2.205 | −0.9025 | −0.8440 | −0.8818 | −0.7296 |
| 9 | I (μ2) | 0.007 | 2.183 | −0.9603 | −0.9449 | −0.9021 | −0.8769 |
| 10 | J (μ2) | 0 | 2.190 | −0.9611 | −0.9238 | −0.9428 | −0.7954 |
| 11 | K (μ3) | 13.45 | 2.202 | −1.1089 | −0.9283 | −0.9950 | −0.9731 |
| 12 | L (μ2) | 20.83 | 2.193 | −0.9177 | −0.8560 | −0.8593 | −0.7460 |
| 13 | M (η) | 40.908 | 2.197 | −0.7537 | −0.7113 | −0.7519 | −0.6610 |
| 14 | N (η) | 46.815 | 2.202 | −0.7887 | −0.7025 | −0.7447 | −0.6628 |
| 15 | O (η) | 41.19 | 2.202 | −0.7528 | −0.7067 | −0.7517 | −0.6531 |
| 16 | P (η) | 50.725 | 2.209 | −0.7387 | −0.6770 | −0.7230 | −0.6295 |
| 17 | Q (η) | 49.693 | 2.207 | −0.7419 | −0.6843 | −0.7155 | −0.6378 |
The relative energies of the protonated isomers at PBE0/def2-tzvp were found to vary by ca. 51 kcal mol−1 between the lowest and highest energy sites. The computations reveal the preferred loci of protonation as being the μ2-sites I and J, which are the only two sites that bridge one Zn and one W atom. These sites differ only by ca. 7 cal mol−1 in energy, with J being the lower energy site. Site K, which is a μ3 site connecting two Zn atoms and one W atom, lies higher in energy by ca. 13 kcal, which is lower than the μ2 W–O–W sites in the molecule, which typically lie 17–22 kcal mol−1 above the J site in energy. The terminal sites range from 41–51 kcal mol−1 above the J site in energy. While the accuracy and precision of the computational approach is likely to be fairly low, given the poly-anionic nature of the molecule and not accounting for specific solvent interactions such as hydrogen bonding or ion pairing in the computations, these energy differences are large enough to be significant. The loci I and J are thus likely to be the most important sites of protonation. This is consistent with general experimental observations of polyoxometalates, in that μ2 oxygen sites protonate preferentially over terminal (η) oxygen sites.
The computed relative energies and bond distances depend to varying extents on the choice of basis set. For bridging (μ2 and μ3) sites, both PBE0/def2-svp and PBE0/def2-tzvp give very relative energies that are typically higher by ca. 2 kcal mol−1 when the smaller basis set it used, whereas the relative energies for the terminal sites differ by 4–5 kcal mol−1 (Fig. 2). In spite of these difference, either level of theory locates the correct locus of protonation, which opens up the possibility for the investigation of larger complexes.
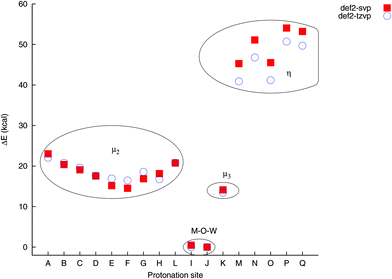 | ||
| Fig. 2 Comparison of relative isomer energies as a function of protonation site and basis set. The energies are only noticeably basis-set dependent for the η-O sites. | ||
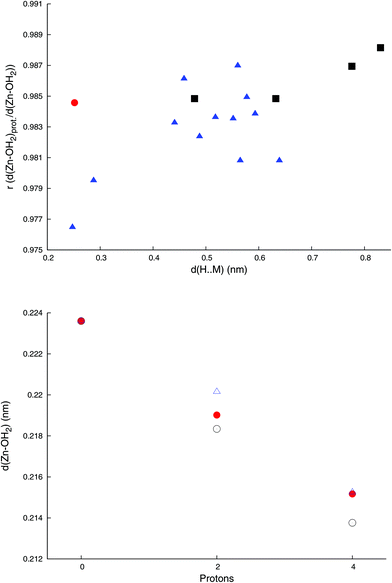
Simultaneous protonation by two protons reduces the surface charge from ca −0.2 C m−2 by ca. 0.04 C m−2, and – apart from a larger general contraction caused by the change in charge – leads to a contraction of the Zn–OH2 bond (Fig. 3), which scales moderately with the distance between the Zn–OH2 bond and the locus of protonation.
While PBE0/def2-tzvp predicts longer bond distances than PBE0/def2-svp for Zn–OH2 in the protonated complexes, both methods predict similar fractional Zn–OH2 bond distances relative to the unprotonated complexes. Thus, either level of theory captures the same general effect of protonation (see ESI, Fig. S2†), with PBE0/def2-tzvp predicting somewhat larger protonation-induced relative contractions in Zn–OH2 bond distances  than PBE0/def2-svp (0.985–0.990). While in this study we have been able to do all the computations at the PBE0/def2-tzvp level of theory, it would thus be justifiable to explore larger systems using PBE0/def2-svp with similar qualitative results.
than PBE0/def2-svp (0.985–0.990). While in this study we have been able to do all the computations at the PBE0/def2-tzvp level of theory, it would thus be justifiable to explore larger systems using PBE0/def2-svp with similar qualitative results.
Protonation at the identified loci, sites I and J, causes the contraction of the Zn–OH2 bond from 2.236 Å to 2.183 Å (site I) and 2.190 Å (site J). Adding a further two protons, so that all I or J sites are protonated, causes the Zn–OH2 bond to further contract (Fig. 3) to 2.138 and 2.152 Å, respectively. The energy difference between these two states is ca. 0.2 kcal, which is likely less than the precision of the computational method.
The correlation between relative energies of the protonated isomers and computed partial charges of the different oxygen sites using a few different schemes was also explored at both pbe0/def2-svp and pbe0/def2-tzvp. As expected from the large difference in isomer energies corresponding to protonation of η and μ2 oxygen sites, and these two groups differing substantially in terms of partial charges, the different partial charges schemes correlate quite well with the computed energies (Fig. 4 (top)). However, at pbe0/def2-svp all investigated schemes predict that the most negative oxygen site is site K, which is not the energetically most favoured site of protonation (see ESI, Fig. S3†). This discrepancy seems to be a general phenomenon, where e.g. NBO charges for the [Nb10O28]6− ion indicates that the most electronegative oxygen site is a μ3-O atom,26 whereas energetic calculations reveal the locus of protonation to lie elsewhere.3
The partial charges computed by in particular MKUFF and HLYGAt are, however, basis set dependent, and decrease with increasing basis set size, whereas the NBO charges vary very little. Intriguingly, at pbe0/def2-tzvp MKUFF gives a more negative partial charge for the I-site than the K-site. Apart from not being helpful in identifying sites I and J as the loci of protonation, this basis set dependence further suggests that these partial charges partitioning methods are not reliable in determining the locus of protonation in metal oxide clusters.
The molecular electrostatic potential (MEP) map (Fig. 4 (bottom)) also identifies the W–O–Zn oxygen sites as having the most negative potential, and appears to indicate that sites I and J have more negative potential than K, which is in accordance with the energetic computations. However, identifying the exact locus based on the MEP map can be challenging, and in itself provides primarily a qualitative view, although it suggests a computationally less demanding approach to gaining an appreciation for where protonation may occur. The chief advantage of the MEP approach is then that it succeeds in separating sites I and J from the other μ2 sites, but it fails to conclusively identify sites I and J over site K as the protonation loci.
The protonation state of the ions in this study at a given pH is not known as only the change in state is measured through titrimetry, it is likely that they are unprotonated above pH 7 given the pKas of ditungstic acid, which are ca. 3.5 and 4.6, respectively.27 The protonation event that is identified through titration, based on its pKa thus probably corresponds to that of addition of two protons to the unprotonated ion, and the likely locus is either site I or J. It is also possible that the protonated system consists of a Boltzmann distributed range of species where sites I and J are protonated to different extents – the precision of the calculations here are not sufficient to rule this in or out.
As part of a study on the rates of aquo-ligand exchange in monomeric coordination complexes of Co(II) and Mn(II), Acharya et al. compared M–OH2 (M = Mn(II), Co(II)) distances with literature data for rates of water exchange.28 While rates of exchange for Co(II) and seven-coordinate Mn(II) complexes do not correlate strongly, or at all, with the M–OH2 bond distance, there is a strong correlation for six-coordinate Mn(II) complexes (Fig. 5). Similarly, rates of exchange were found to correlate with the number of 1,10-phenanthroline or 2,2′-bipyridyl ligands in Mn(II) complexes, but not in Co(II) complexes.
 | ||
| Fig. 5 Rates of water exchange vs. M–OH2 distances for Mn(II), Fe(III), Co(II) and Ni(II). See Acharya et al.28, Balogh et al.29 and ESI† for data and references. | ||
In light of the observed rate vs. bond-distance dependence in Mn(II) complexes in the literature, and lack thereof in Co(II) complexes, it is not surprising that a process that leads to M–OH2 bond contraction, such as protonation, then affects [Mn4(H2O)2(P2W15O56)2]16− different to [Co4(H2O)2(P2W15O56)2]16−, although it in itself does not explain the underlying mechanism. It does, however, already suggest an approach to predicting which Wells–Dawson derivatives will exhibit weak vs. strong pH-dependent rates of exchange. The lack of correlation for hepta-coordinate Mn(II) further supports the mechanism being interchange associative for the hexa-coordinate Mn(II), in line with the general view.30 Likewise, the lack of strong correlation for Co(II) is consistent with the interchange pathway generally accepted for this ion. What is important to note, however, is that although these models were developed for monomeric complexes, they are often assumed to be applicable to more complex systems, but it is rarely possible to conclusively confirm this.
Based exclusively on the agreement of the rate behaviours of the polyoxometalates in this study, with those of monomeric complexes in the literature involving the same Mn(II) and Co(II) centres, we suggest that the aquo-ligand exchange in the Mn(II) polyoxometalate follows an interchange associative pathway and that it follows an interchange dissociative pathway for Co(II).
The microscopic view of the reaction mechanisms in the present case is not clear, however. In the case of a suggested interchange associative mechanism for [Mn4(H2O)2(P2W15O56)2]16−, the Mn(II) is situated in a fairly rigid site where such a mechanism is would have been less anticipated. However, the computed protonation-induced bond distance change for the Zn(II) complex, −0.05 Å, suggests a ca. ten-fold rate increase when compared with the plot for Mn(II) complexes (Fig. 5), which is in good agreement with the observed ca. 15-fold rate increase. Similar plots for octahedral Ni(II) and Fe(III) complexes from the literature suggests that Fe(III) will show a significant decrease in rates of exchange with pH and decreasing bond distance, whereas the behaviour of Ni(II) is more difficult to predict. We do not want to speculate on the exact mechanism of the Co(II) polyoxometalate, other than to state that while it appears counter-intuitive that a shortening of the bond-distance of an exchanging ligand should not affect the rate of exchange, the literature data is quite conclusive in demonstrating the lack of correlation between Co(II)–OH2 bond distances and rates of aquo-ligand exchange.
Conclusions
Prompted by the lack of pH-dependence of the rate of exchange of the aquo-ligand in [Co4(H2O)2(P2W15O56)2]16− and the strong pH dependence of the same type of exchange in [Mn4(H2O)2(P2W15O56)2]16− – in spite of their similar protonation profile – we have located the sites of protonation in a nanometre-sized sandwich polyoxometalate using energetic computations. Alternative approaches to locating protonation sites have also been investigated, but only direct computation of the relative energies of the different protonated isomers appears to be reliable. The loci of protonation were found to be sites I and J, which are two Zn–O–W oxygen sites.Protonation induces a contraction of the Zn–OH2 bond distance which scales directly with increased protonation of sites I and J, as well as moderately with the distance between the Zn–OH2 zinc atom and the site of protonation, although the change in charge of the molecule is responsible for the majority of contraction effect.
A literature comparison between crystallographically determined bond distances and rates of aquo-ligand exchange in a series of complexes revealed that the rate of aquo-ligand exchange in monomeric Mn(II) complexes, which undergo interchange associative exchange, is bond distance dependent, whereas it is not for monomeric Co(II) complexes, which undergo interchange dissociative exchange. Because the observed increase in the rate of exchange of the Mn(II) polyoxometalate correlates well with that which would be caused by the computed Zn–OH2 shortening, and because the rates of exchange in the Co(II) polyoxometalates is not affected by the expected change in bond distance, we suggest that this implies that the mechanisms of aquo-ligand exchange in the polyoxometalates in this study may be the same as for the monomeric Co(II) and Mn(II) complexes.
Finally, we here show through protonation of a wide range of sites in the [Zn4(H2O)2(PW9O34)2]10− ion that the metal–aquo-ligand bond contraction depends on the distance between the protonation sites and the metal–aquo ligand even when that distance exceeds 0.75 Å (Fig. 3), which has significant implications for the modelling and understanding of extended surfaces such as minerals. Fragments used either computationally or conceptually must thus be large enough to capture the essential chemistry, or key aspects of the mechanism, such as protonation, may be overlooked.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is dedicated to the memory of the late Professor Leone Spiccia. MPB and CAO acknowledge generous support from the Kempe Foundation (JCK-1719). Computational resources were in provided by the High Performance Computing Center North (HPC2N) via grant SNIC 2018/3-253.References
- Y.-F. Song and R. Tsunashima, Chem. Soc. Rev., 2012, 41, 7384–7402 RSC.
- S.-S. Wang and G.-Y. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef PubMed.
- J. R. Rustad and W. H. Casey, Nat. Mater., 2012, 11, 223–226 CrossRef PubMed.
- W. H. Casey, Environ. Chem., 2015, 12, 1–19 CrossRef.
- W. H. Casey and J. R. Rustad, New J. Chem., 2016, 40, 898–905 RSC.
- C. J. Gomez-Garcia, J. J. Borras-Almenar, E. Coronado and L. Ouahab, Inorg. Chem., 1994, 33, 4016–4022 CrossRef.
- X. Zhang, Q. Chen, D. C. Duncan, C. F. Campana and C. L. Hill, Inorg. Chem., 1997, 36, 4208–4215 CrossRef.
- R. G. Finke and M. W. Droege, Inorg. Chem., 1983, 22, 1006–1008 CrossRef.
- R. G. Finke, M. W. Droege and P. J. Domaille, Inorg. Chem., 1987, 26, 3886–3896 CrossRef.
- Q. Yin, J. M. Tan, C. Besson, Y. V. Geletii, D. G. Musaev, A. E. Kuznetsov, Z. Luo, K. I. Hardcastle and C. L. Hill, Science, 2010, 328, 342–345 CrossRef PubMed.
- C. A. Ohlin, S. J. Harley, J. G. McAlpin, R. K. Hockin, B. Q. Mercado, R. L. Johnson, E. M. Villa, M. K. Fidler, M. M. Olmstead, L. Spiccia, R. D. Britt and W. H. Casey, Chem. – Eur. J., 2011, 17, 4408–4417 CrossRef PubMed.
- D. Lieb, A. Zahl, E. F. Wilson, C. Streb, L. C. Nye, K. Meyer and I. Ivanovi-Burmazovi, Inorg. Chem., 2011, 50, 9053–9058 CrossRef PubMed.
- T. J. Swift and R. E. Connick, J. Chem. Phys., 1962, 37, 307–320 CrossRef.
- T. J. Swift and R. E. Connick, J. Chem. Phys., 1964, 41, 2553–2554 CrossRef.
- L. Ruhlmann, L. Nadjo, J. Canny, R. Contant and R. Thouvenot, Eur. J. Inorg. Chem., 2002, 2002, 975–986 CrossRef.
- S. Romo, J. A. Fernández, J. M. Maestre, B. Keita, L. Nadjo, C. de Graaf and J. M. Poblet, Inorg. Chem., 2007, 46, 4022–4027 CrossRef PubMed.
- R. Sharma, J. Zhang and C. A. Ohlin, Dalton Trans., 2015, 44, 19068–19071 RSC.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6159 CrossRef.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3093 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Revision B.01, Gaussian Inc., Wallingford CT, 2016 Search PubMed.
- E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales, C. R. Landis and F. Weinhold, NBO 6.0, http://nbo6.chem.wisc.edu/ Search PubMed.
- B. H. Besler, K. M. Merz and P. A. Kollman, J. Comput. Chem., 1990, 11, 431–439 CrossRef.
- C. M. Breneman and K. B. Wiberg, J. Comput. Chem., 1990, 11, 361–373 CrossRef.
- H. Hu, Z. Lu and W. Yang, J. Chem. Theory Comput., 2007, 3, 1004–1013 CrossRef PubMed.
- S. Hayashi, S. Yamazoe, K. Koyasu and T. Tsukuda, RSC Adv., 2016, 6, 16239–16242 RSC.
- G. Schwarzenbach, G. Geier and J. Littler, Helv. Chim. Acta, 1962, 45, 2601–2612 CrossRef.
- S. S. Acharya, B. Winther-Jensen, L. Spiccia and C. A. Ohlin, Aust. J. Chem., 2017, 70, 751–754 CrossRef.
- E. Balogh, A. M. Todea, A. Müller and W. H. Casey, inorg. Chem., 2007, 46, 7087–7092 CrossRef PubMed.
- Y. Ducommun, K. E. Newman and A. E. Merbach, Inorg. Chem., 1980, 19, 3696–3703 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Optimised structures, computed energies, partial charges and molecular electrostatic surface. See DOI: 10.1039/C8DT02342E |
| This journal is © The Royal Society of Chemistry 2018 |