Computational chemical analysis of Eu(iii) and Am(iii) complexes with pnictogen-donor ligands using DFT calculations†
Abstract
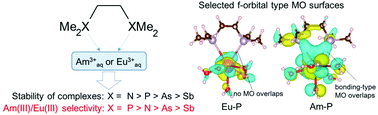
We demonstrated density functional calculations of Eu(III) and Am(III) complexes with pnictogen-donor (X) ligands, (CH3)2X–CH2–CH2–X(CH3)2 (X = N, P, As and Sb). We investigated the optimized structures of the complexes and the Gibbs energy differences in the complex formation reactions. The results indicated that the N- and P-donor ligands exhibit Am(III) ion selectivity over Eu(III) ions; especially, the P-donor ligand showed the highest selectivity. The tendency of the Am(III)/Eu(III) selectivity by the pnictogen-donor ligands was found to be comparable to that of the soft acid classification in the hard and soft acids and bases rule. Mulliken's spin population analysis indicated that the bonding properties between the metal ion and the pnictogen atoms correlated with the Am(III)/Eu(III) selectivity. In particular, the participation of f-orbital electrons of the metal ion in the covalency was indicated to play an important role in the selectivity.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...