Novel chelators based on adamantane-derived semicarbazones and hydrazones that target multiple hallmarks of Alzheimer's disease†
Abstract
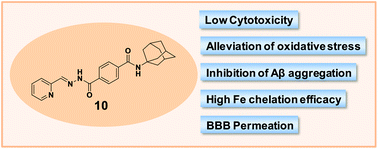
Alzheimer's disease (AD) is characterized by multiple pathological hallmarks, including β-amyloid aggregation, oxidative stress, and metal dys-homeostasis. In the absence of treatments addressing its multi-factorial pathology, we designed novel multi-functional adamantane-based semicarbazones and hydrazones (1–12) targeting AD hallmarks. Of these, 2-pyridinecarboxaldehyde (N-adamantan-1-yl)benzoyl-4-amidohydrazone (10) was identified as the lead compound, which demonstrated: (1) pronounced iron chelation efficacy; (2) attenuation of CuII-mediated β-amyloid aggregation; (3) low cytotoxicity; (4) inhibition of oxidative stress; and (5) favorable characteristics for effective blood–brain barrier permeation. Structure–activity relationships revealed that pyridine-derived hydrazones represent a promising pharmacophore for future design strategies due to their ability to bind critical FeII pools. Collectively, the unique multi-functional activity of these agents provides a novel therapeutic strategy for AD treatment.


 Please wait while we load your content...
Please wait while we load your content...