Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Through-conjugation of two phosphaalkyne (‘C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e002.gif) P’) moieties mediated by a bimetallic scaffold †
P’) moieties mediated by a bimetallic scaffold †
Matthew. C.
Leech
 and
Ian R.
Crossley
and
Ian R.
Crossley
 *
*
Department of Chemistry, University of Sussex, Brighton, UK. E-mail: i.crossley@sussex.ac.uk; Fax: +44 (0)1273876678; Tel: +44 (0)1273 877302
First published on 28th February 2018
Abstract
Through-conjugation of two phosphaalkyne moieties within an isolable molecule is demonstrated for the first time with the synthesis of [{Ru(dppe)2}2{μ-(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H4-p}(C
C)2C6H4-p}(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P)2], via base-induced desilylation of [{Ru(dppe)2}2{μ-(C
P)2], via base-induced desilylation of [{Ru(dppe)2}2{μ-(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H4-p}(η1-P
C)2C6H4-p}(η1-P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3)2]2+. The nature of the cyaphide ligands and their influence upon the bimetallic core are studied electrochemically.
CSiMe3)2]2+. The nature of the cyaphide ligands and their influence upon the bimetallic core are studied electrochemically.
Phosphaalkynes (RC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P)1 are archetypal models of the phosphorus–carbon analogy,2 being both isolobal and isoelectronic with alkynes. Though dichotomous in nature – by virtue of the polarity and lone-pair imparted by phosphorus – their chemical analogy to alkynes is well-established, with a prevalence of cycloaddition/oligomerisation reactions, while both η2-CP (cf. alkynes) and η1-P (cf. nitriles, alkynyls) complexes with transition metals are known.3 Notwithstanding, an enduring omission lies with the incorporation of the discrete ‘C
P)1 are archetypal models of the phosphorus–carbon analogy,2 being both isolobal and isoelectronic with alkynes. Though dichotomous in nature – by virtue of the polarity and lone-pair imparted by phosphorus – their chemical analogy to alkynes is well-established, with a prevalence of cycloaddition/oligomerisation reactions, while both η2-CP (cf. alkynes) and η1-P (cf. nitriles, alkynyls) complexes with transition metals are known.3 Notwithstanding, an enduring omission lies with the incorporation of the discrete ‘C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P’ moiety into architectures featuring extended conjugation (cf. the prevalence of polyacetylides), a desirable target – particularly from an organometallic standpoint4 – given extensive interest in acetylenic and phosphorus-containing moieties in the context of developing molecular electronic components.5–7 Indeed, the conjugation of phosphaalkyne (‘C
P’ moiety into architectures featuring extended conjugation (cf. the prevalence of polyacetylides), a desirable target – particularly from an organometallic standpoint4 – given extensive interest in acetylenic and phosphorus-containing moieties in the context of developing molecular electronic components.5–7 Indeed, the conjugation of phosphaalkyne (‘C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P’) moieties with other π-systems is limited to the small range of aromatic phosphaalkynes: PhC
P’) moieties with other π-systems is limited to the small range of aromatic phosphaalkynes: PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P,8 2,6-R-C6H3C
P,8 2,6-R-C6H3C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P (R = Mes, tBu),9 2,6-R-4-R′-C6H2C
P (R = Mes, tBu),9 2,6-R-4-R′-C6H2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P (R = tBu, R′ = OMe, NMe2;9b R=R′ = tBu,10 CMe2Et11) and the putative P
P (R = tBu, R′ = OMe, NMe2;9b R=R′ = tBu,10 CMe2Et11) and the putative P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) E (E = CH, N,12a,b P12c–e), which were generated (transiently) and observed in the gas phase. The latter (P
E (E = CH, N,12a,b P12c–e), which were generated (transiently) and observed in the gas phase. The latter (P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P) is also among a very limited range of compounds to feature two ‘C
P) is also among a very limited range of compounds to feature two ‘C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P’ moieties (Chart 1),13 and is the sole precedent example for which their mutual conjugation might reasonably be invoked (albeit unstudied).
P’ moieties (Chart 1),13 and is the sole precedent example for which their mutual conjugation might reasonably be invoked (albeit unstudied).
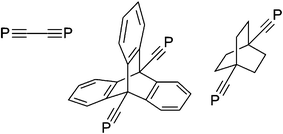 | ||
| Chart 1 Known bis-phosphaalkynes.12,13 | ||
Though a small number of transition metal complexes featuring trans-disposed η1-phosphaalkynes has been reported,14viz. [M(L)2(P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)2] (M = Mo, L = dppe, depe, R2PC2H4PR2, R = Tol, ClC6H4); (M = W, L = dppe), [Mo(depe)2(P
CtBu)2] (M = Mo, L = dppe, depe, R2PC2H4PR2, R = Tol, ClC6H4); (M = W, L = dppe), [Mo(depe)2(P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAd)2] and [Mo(dppe)2(P
CAd)2] and [Mo(dppe)2(P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3)2],15 even the concept of metal-mediated conjugation (cf. bis-alkynyl complexes) was unexplored prior to our recent report of the unprecedented cyaphide–alkynyl complexes trans-[Ru(dppe)2(C
CSiMe3)2],15 even the concept of metal-mediated conjugation (cf. bis-alkynyl complexes) was unexplored prior to our recent report of the unprecedented cyaphide–alkynyl complexes trans-[Ru(dppe)2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)(C
CR)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P)] (R = CO2Me, p-An).16 Herein, we extend this conceptual framework to consider, for the first time, extended conjugation between multiple ‘C
P)] (R = CO2Me, p-An).16 Herein, we extend this conceptual framework to consider, for the first time, extended conjugation between multiple ‘C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P’ moieties, mediated by a bimetallic, redox-active, core; we also elucidate the electronic and redox nature of these complexes.
P’ moieties, mediated by a bimetallic, redox-active, core; we also elucidate the electronic and redox nature of these complexes.
The sequential treatment of the bisethynylbenzene-bridged bimetallic complex [{Ru(dppe)2}2{μ-(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H4-p}Cl2] (1) with two equivalents of AgOTf and P
C)2C6H4-p}Cl2] (1) with two equivalents of AgOTf and P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3 facilitates installation of two terminal phosphaalkyne moieties to afford 22+ (Scheme 1). Formation of 22+ is evident from characteristic spectroscopic signatures indicative of a coordinated phosphaalkyne (δP 111.4, JPP 34 Hz) in proximity to the dppe scaffold (δP 42.2 (1
CSiMe3 facilitates installation of two terminal phosphaalkyne moieties to afford 22+ (Scheme 1). Formation of 22+ is evident from characteristic spectroscopic signatures indicative of a coordinated phosphaalkyne (δP 111.4, JPP 34 Hz) in proximity to the dppe scaffold (δP 42.2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio)), while the carbon-rich bridge remains apparent from 13C{1H} NMR and infrared (νC
4 ratio)), while the carbon-rich bridge remains apparent from 13C{1H} NMR and infrared (νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C 2054 cm−1) spectroscopic data. Retention of the silyl moieties follows from heteronuclear (1H–29Si) correlation, while the triflate counter-ion is observed in the 19F-NMR spectrum (δF −78.9); bulk composition is affirmed by microanalysis.
C 2054 cm−1) spectroscopic data. Retention of the silyl moieties follows from heteronuclear (1H–29Si) correlation, while the triflate counter-ion is observed in the 19F-NMR spectrum (δF −78.9); bulk composition is affirmed by microanalysis.
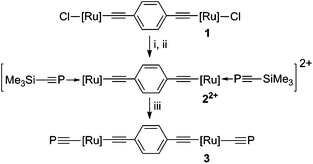 | ||
Scheme 1 Reagents and conditions: (i) CH2Cl2, 2 AgOTf, (ii) 2 P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3 in toluene, 1 h.; (iii) thf, 2 KOtBu, 1 h. [Ru] = Ru(dppe)2. CSiMe3 in toluene, 1 h.; (iii) thf, 2 KOtBu, 1 h. [Ru] = Ru(dppe)2. | ||
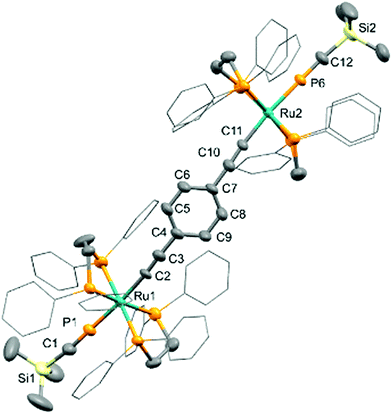
The connectivity of 22+ is further supported by X-ray diffraction data (Fig. 1).17 The internal geometry is largely unremarkable, exhibiting only slight deviations from linearity about the metal centres (∠ P–Ru–C 173.4(2), 175.3(2)°) and in the bridge (∠ Ru–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C 174.5(4), 174.2(4); ∠ C
C 174.5(4), 174.2(4); ∠ C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C 174.5(5), 172.7(5)°) characteristic, respectively, of other bis-alkynyls18 and the limited range of structurally characterized complexes comprising the ‘Ru2{μ-(C
C–C 174.5(5), 172.7(5)°) characteristic, respectively, of other bis-alkynyls18 and the limited range of structurally characterized complexes comprising the ‘Ru2{μ-(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H4-p}’ and related cores.19 The coordinated phosphaalkyne moieties are similarly consistent with related analogues.14–16,20
C)2C6H4-p}’ and related cores.19 The coordinated phosphaalkyne moieties are similarly consistent with related analogues.14–16,20
Conversion of the η1-P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3 moieties to terminal cyaphide ligands (‘–C
CSiMe3 moieties to terminal cyaphide ligands (‘–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P’) proceeds upon treating 22+ with 2 equiv. KOtBu,21 affording 3 in moderate yield (Scheme 1). While single crystals of 3 can be grown, their rapid desolvation during mounting (even at low temperature) has precluded the acquisition of X-ray diffraction data. Nonetheless, the identity of 3 is readily established from the characteristic spectroscopic features and changes that accompany the desilylative rearrangement of η1-P
P’) proceeds upon treating 22+ with 2 equiv. KOtBu,21 affording 3 in moderate yield (Scheme 1). While single crystals of 3 can be grown, their rapid desolvation during mounting (even at low temperature) has precluded the acquisition of X-ray diffraction data. Nonetheless, the identity of 3 is readily established from the characteristic spectroscopic features and changes that accompany the desilylative rearrangement of η1-P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3 to cyaphide;16,20aviz. (i) reduction in frequency of the C
CSiMe3 to cyaphide;16,20aviz. (i) reduction in frequency of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P stretch (ΔνC
P stretch (ΔνC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P ∼ −12 cm−1); (ii) loss of NMR resonances for silyl and −OTf moieties; (iii) increase in frequency (ΔδP 48) for the phosphaalkynic P-centres, with reduced magnitude of the PCP–Pdppe coupling (precluding its resolution); (iv) increased frequency (Δδc 92) for the cyaphidic carbon resonance, consistent with formation of an organometallic linkage (cf M–CO, M–CN). These data compare well with those we have noted previously16 and those for Grutzmacher's seminal complex [RuH(dppe)2(C
P ∼ −12 cm−1); (ii) loss of NMR resonances for silyl and −OTf moieties; (iii) increase in frequency (ΔδP 48) for the phosphaalkynic P-centres, with reduced magnitude of the PCP–Pdppe coupling (precluding its resolution); (iv) increased frequency (Δδc 92) for the cyaphidic carbon resonance, consistent with formation of an organometallic linkage (cf M–CO, M–CN). These data compare well with those we have noted previously16 and those for Grutzmacher's seminal complex [RuH(dppe)2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P)];20a they also concur with data calculated for 3 using the PBE functional (Table 1).
P)];20a they also concur with data calculated for 3 using the PBE functional (Table 1).
| δ P(CP) | ΔδP(CP)b | δ C(CP) | ΔδC(CP)b | |
|---|---|---|---|---|
a {Ru} = Ru(dppe)2.
b Δδ on conversion from η1-P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR to terminal cyaphide.
c Increase in δP due to SiPh3vs. SiMe3.
d GIAO method with the PBE functional (lanl2dz for Ru; 6-31G** for all other atoms); referenced to H3PO4 or Me4Si at the same level of theory. CR to terminal cyaphide.
c Increase in δP due to SiPh3vs. SiMe3.
d GIAO method with the PBE functional (lanl2dz for Ru; 6-31G** for all other atoms); referenced to H3PO4 or Me4Si at the same level of theory.
|
||||
| 22+ | 111.4 | — | 189.8 | — |
| 3 | 159.7 | 48.3 | 281.8 | 92.0 |
[{Ru}(C2R)(P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3)]+ CSiMe3)]+ |
108.4 | — | 192.6 | — |
[{Ru}(C2R)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P)] (R = CO2Me) P)] (R = CO2Me) |
168.5 | 60.0 | 279.1 | 86.5 |
[{Ru}(C2R)(P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3)]+ CSiMe3)]+ |
112.8 | — | 188.2 | — |
[{Ru}(C2R)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P)] (R = p-An) P)] (R = p-An) |
159.5 | 46.7 | 281.9 | 93.7 |
[{Ru}H(P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiPh3)]+ CSiPh3)]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20a 20a |
143.8c | — | 175.1 | — |
[{Ru}H(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P)]20a P)]20a |
165.0 | 21.3 | 287.1 | 112.0 |
| 22+ (calc)d | 118.4 | — | 188.8 | — |
| 3 (calc)d | 166.4 | 48.0 | 271.4 | 82.6 |
The optimized gas-phase geometries of 22+ and 3 (see ESI†)22 both exhibit slightly greater linearity about the metal centres and bridge when compared with the solid-state structure of 22+, alongside marginally longer C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P linkages (∼1.58 Å). These features are consistent with a prevalence of packing effects in the solid state, as noted previously for several η1-P
P linkages (∼1.58 Å). These features are consistent with a prevalence of packing effects in the solid state, as noted previously for several η1-P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR complexes,20,23 and for our precedent cyaphide–alkynyls.16 The calculated C
CR complexes,20,23 and for our precedent cyaphide–alkynyls.16 The calculated C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P stretching mode for 3 (asym. νC
P stretching mode for 3 (asym. νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P 1224 cm−1) also compares well with experiment (νC
P 1224 cm−1) also compares well with experiment (νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P 1247 cm−1). Notably, the experimentally observed frequency reflects a slightly stronger C
P 1247 cm−1). Notably, the experimentally observed frequency reflects a slightly stronger C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P linkage for 3 than in [RuH(dppe)2(C
P linkage for 3 than in [RuH(dppe)2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P)] (νC
P)] (νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P 1239 cm−1),20a attributable to competition with the trans-alkynyl for Ru → π* donation. Indeed, we noted this previously for cyaphide–alkynyls, though to a greater extent (νC
P 1239 cm−1),20a attributable to competition with the trans-alkynyl for Ru → π* donation. Indeed, we noted this previously for cyaphide–alkynyls, though to a greater extent (νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P 1255, 1260 cm−1),16 suggesting a reduced competition within the bimetallic scaffold.
P 1255, 1260 cm−1),16 suggesting a reduced competition within the bimetallic scaffold.
The frontier orbitals of 22+ and 3 (Fig. 2) show similarities, the HOMO in each case being dominated by the bridging π-system (76%, 22+; 54% 3) with a modest contribution from the metals (14% 22+; 26% 3). Notably, the HOMO of 3 also includes contributions from πC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P (14%), which engage in out-of-phase mixing with the Ru (dxy, dxz), πC
P (14%), which engage in out-of-phase mixing with the Ru (dxy, dxz), πC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and πAr orbitals, consistent with some level of through-conjugation. The contributions from πC
C and πAr orbitals, consistent with some level of through-conjugation. The contributions from πC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P increase appreciably in the mutually degenerate HOMO−1 and HOMO−2 (∼25%, see ESI†), lying 0.36 eV below the HOMO, albeit without involvement of the bridging arene (1%). In marked contrast, there is negligible contribution (<10%) from the η1-P
P increase appreciably in the mutually degenerate HOMO−1 and HOMO−2 (∼25%, see ESI†), lying 0.36 eV below the HOMO, albeit without involvement of the bridging arene (1%). In marked contrast, there is negligible contribution (<10%) from the η1-P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3 moieties of 22+ to any occupied frontier orbitals, their involvement becoming significant only in the appreciably stabilized HOMO−3 and HOMO−4, lying ca. 1.4 eV below the HOMO. Finally, in respect of 3, we note that the terminal cyaphidic lone-pairs manifest in the HOMO−14 and HOMO−15, being stabilised by ca. 2 eV relative to the HOMO. This is entirely consistent with expectation, being similar to our previous observations,16 and those for phosphaalkynes more generally.24 Additionally, NBO calculations suggest these to reside in orbitals of ca. 75% s and 25% p character, as is typical of phosphaalkynes.
CSiMe3 moieties of 22+ to any occupied frontier orbitals, their involvement becoming significant only in the appreciably stabilized HOMO−3 and HOMO−4, lying ca. 1.4 eV below the HOMO. Finally, in respect of 3, we note that the terminal cyaphidic lone-pairs manifest in the HOMO−14 and HOMO−15, being stabilised by ca. 2 eV relative to the HOMO. This is entirely consistent with expectation, being similar to our previous observations,16 and those for phosphaalkynes more generally.24 Additionally, NBO calculations suggest these to reside in orbitals of ca. 75% s and 25% p character, as is typical of phosphaalkynes.
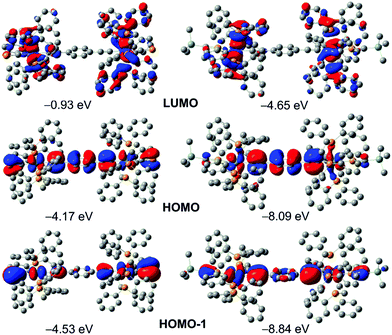 | ||
| Fig. 2 Frontier orbitals for 3 (left) and 22+ (right), with relative energies (see also ESI†). | ||
As is typical of complexes with the Ru(dppe)2 scaffold, the latter dominates the virtual orbitals of 3, which are mostly centred on the dppe ligands; the bridge contributes marginally to LUMO+12 and LUMO+14, lying 4 eV above the HOMO. In contrast, while the LUMO/LUMO+1 of 22+ are again dominated by the Ru(dppe)2 framework, LUMO+2 is centred on the unsaturated core, with appreciable contributions from π*C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P (60%) and the bridge (15%). This is reflected in the electronic spectrum of 22+, assigned in comparison with those derived from TD-DFT studies,25 calculating the first 200 excited states. This offers a fair approximation of the observed UV spectra for 22+ and 3 (within limitations of the model), providing sufficient correlation to assist in the assignment of some key features. Thus, a feature at 350 nm (28
P (60%) and the bridge (15%). This is reflected in the electronic spectrum of 22+, assigned in comparison with those derived from TD-DFT studies,25 calculating the first 200 excited states. This offers a fair approximation of the observed UV spectra for 22+ and 3 (within limitations of the model), providing sufficient correlation to assist in the assignment of some key features. Thus, a feature at 350 nm (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 cm−1) includes significant contribution from LLCT bands (πC
571 cm−1) includes significant contribution from LLCT bands (πC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C → π*Ar and πC
C → π*Ar and πC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C → π*C
C → π*C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P) with marginal involvement of intraligand CT (πC
P) with marginal involvement of intraligand CT (πC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C → π*C
C → π*C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), alongside the dominant MLCT and LLCT associated with excitation from the HOMO/HOMO+1 to low-lying dppe-based orbitals. A second feature around 260 nm (38
C), alongside the dominant MLCT and LLCT associated with excitation from the HOMO/HOMO+1 to low-lying dppe-based orbitals. A second feature around 260 nm (38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 462 cm−1) is primarily composed of ILCT within the dppe scaffold (<HOMO−10 → LUMO), but with additional contribution from πC
462 cm−1) is primarily composed of ILCT within the dppe scaffold (<HOMO−10 → LUMO), but with additional contribution from πC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P → π*C
P → π*C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P ILCT and πAr → π*C
P ILCT and πAr → π*C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P LLCT (HOMO−3 → LUMO+5). In contrast, features in the UV/Vis spectrum of 3 around 370 nm (27
P LLCT (HOMO−3 → LUMO+5). In contrast, features in the UV/Vis spectrum of 3 around 370 nm (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 cm−1) and 250 nm (40
027 cm−1) and 250 nm (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) are wholly dominated by MLCT and LLCT transitions to the dppe scaffold, with marginal contributions from ILCT within the bridging π-framework; contributions from transitions to the high-lying π*C
000 cm−1) are wholly dominated by MLCT and LLCT transitions to the dppe scaffold, with marginal contributions from ILCT within the bridging π-framework; contributions from transitions to the high-lying π*C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P (LUMO+36 to LUMO+39) are negligible.
P (LUMO+36 to LUMO+39) are negligible.
The redox behaviours of 22+ and 3 were explored using cyclic voltammetry (Table 2 and ESI†), both compounds exhibiting two distinct oxidative events, which can be assigned (trivially26) to sequential generation of the RuIII/RuII and RuIII/RuIII species. For 22+ an initial quasi-reversible oxidation occurs at significantly more anodic potential than the corresponding (reversible) feature of 1, presumably a corollary of its cationic nature. The second (irreversible) oxidation is similarly shifted to more positive potential,27 and demonstrates an appreciable stability for the mixed valence state [22+]+, Kc being comparable in magnitude to that of [1]+ and related terminal alkynyls.19e,28
| E pa/V | E pc/V | E 1/2(ΔEpp)/V | ΔEpa/V |
K
c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|
|---|---|---|---|---|---|
| a CH2Cl2/0.1 M [NBu4]PF6 using 1 mM analyte solutions at (25 °C), with Pt disc (1 mm) working electrode, Pt wire counter electrode and Ag wire pseudo-reference at 100 mV s−1. Potentials relative to the FcH/FcH+ couple (0.00 V), referenced using internal Fc*H/Fc*H+ (−0.56 V (Epp 78 mV) vs. Fc/Fc+). b K c = 10ΔE/59 mV at 298 K. c Irreversible oxidation. d Irreversible reduction. | |||||
| 1 | −0.268 | −0.348 | −0.308 (80) | 0.351 | 8.9 × 105 |
| 0.081 | 0.004 | 0.043 (77) | |||
| 22+ | 0.705 | 0.565 | 0.635 (140) | 0.290 | 0.8 × 105 |
| 0.995 | |||||
| 3 | −0.210c | −0.780d | — | 0.190 | 1.7 × 103 |
| −0.020c | |||||
In the case of 3, two irreversible oxidations are observed, the initial event showing a slight anodic shift relative to 1, and indeed related alkynyl systems;19e,28 the second occurs at lower potential than the corresponding oxidation of [1]+. On the reverse scan, an irreversible reduction process is observed at heavily cathodic potential. Notably, the diminished separation of the oxidative events indicates a reduced stability for the mixed valence state ([3]+) in comparison to [1]+ and, indeed, related alkynyl complexes and [22+]+, Kc being two-orders of magnitude lower than for its counterparts.19e,28 Notwithstanding, some stability is apparent, which implies some retention of the electronic coupling characteristic of the “Ru2{μ-(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H4-p}” scaffold, albeit diminished by the seemingly electron-acceptor character of the cyaphide ligand.
C)2C6H4-p}” scaffold, albeit diminished by the seemingly electron-acceptor character of the cyaphide ligand.
Conclusions
In conclusion, we have described the first isolable compound to incorporate two ‘C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P’ moieties as part of the same conjugated scaffold, viz. [Ru2{μ-(C
P’ moieties as part of the same conjugated scaffold, viz. [Ru2{μ-(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H4-p}(C
C)2C6H4-p}(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P)2] (3). The electronic spectrum shows a dominance of LLCT and MLCT transitions from the bridge and phosphacarbon moieties to the dppe scaffold, with negligible ILCT within the π-system. The redox properties of 3 are more interesting and suggest some electron-acceptor character for the cyaphide ligand. While its presence leads to irreversible redox behaviour and serves to destabilize the mixed-valent state [3]+, the retention of electronic coupling within the bimetallic core provides initial conceptual validation for the incorporation of the cyaphide ligand into electro-active complexes. This will require engineering of appropriately stabilizing ancillary scaffolds, a challenge with which we are currently engaged.
P)2] (3). The electronic spectrum shows a dominance of LLCT and MLCT transitions from the bridge and phosphacarbon moieties to the dppe scaffold, with negligible ILCT within the π-system. The redox properties of 3 are more interesting and suggest some electron-acceptor character for the cyaphide ligand. While its presence leads to irreversible redox behaviour and serves to destabilize the mixed-valent state [3]+, the retention of electronic coupling within the bimetallic core provides initial conceptual validation for the incorporation of the cyaphide ligand into electro-active complexes. This will require engineering of appropriately stabilizing ancillary scaffolds, a challenge with which we are currently engaged.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Royal Society, Engineering and Physical Sciences Research Council (EPSRC; EP/N016785/1) and the University of Sussex (studentship to M. C. L.). I. R. C. gratefully acknowledges the award of a Royal Society University Research Fellowship. We thank Dr S. M. Roe (Sussex) for assistance with structural refinement for 22+.Notes and references
- For reviews of phosphacarbon and phosphaalkene chemistry see: (a) F. Mathey, Angew. Chem., Int. Ed., 2003, 42, 1578–1604 CrossRef CAS PubMed; (b) R. Appel, in Multiple Bonds and Low Coordination in Phosphorus Chemistry, ed. M. Regitz and O. J. Scherer, Thieme, Stutgart, 1990 Search PubMed; (c) L. N. Markovski and V. D. Romanenko, Tetrahedron, 1989, 45, 6019–6090 CrossRef; (d) R. Appel, F. Knoll and I. Ruppert, Angew. Chem., Int. Ed. Engl., 1981, 20, 731–744 CrossRef.
- K. B. Dillon, F. Mathey and J. F. Nixon, Phosphorus: The Carbon Copy, Wiley, Chichester, 1998 Search PubMed.
- (a) J. F. Nixon, Coord. Chem. Rev., 1995, 145, 201–258 Search PubMed; (b) J. F. Nixon, Chem. Rev., 1988, 88, 1327–1362 CrossRef CAS.
- See for example and reviews: (a) C. Schubert, J. T. Margrat, T. Clark and D. M. Guidi, Chem. Soc. Rev., 2015, 44, 988–998 RSC; (b) P. J. Low, Dalton Trans., 2005, 2821–2824 RSC; (c) M. I. Bruce and P. J. Low, Adv. Organomet. Chem., 2004, 51, 179–444 CrossRef; (d) M. I. Bruce and P. J. Low, Adv. Organomet. Chem., 2001, 48, 71–288 CrossRef; (e) F. Paul and C. Lapinte, Coord. Chem. Rev., 1998, 178–180, 431–509 CrossRef CAS; (f) I. R. Whittall, A. J. McDonagh and M. G. Humphrey, Adv. Organomet. Chem., 1998, 42, 291–362 CrossRef CAS; (g) N. J. Long, Angew. Chem., Int. Ed. Engl., 1995, 34, 21–38 CrossRef CAS.
- (a) M. A. Shameem and A. Orthaber, Chem. – Eur. J., 2016, 22, 10718–10735 CrossRef CAS PubMed; (b) T. Baumgartner, Acc. Chem. Res., 2014, 47, 1613–1622 CrossRef CAS PubMed; (c) T. Baumgartner and R. Reau, Chem. Rev., 2006, 106, 4681–4727 CrossRef CAS PubMed and references therein.
- (a) X.-L. Geng, Q. Hu, B. Schafer and S. Ott, Org. Lett., 2010, 12, 692–695 CrossRef CAS PubMed; (b) E. Oberg, B. Schafer, X.-L. Geng, J. Pettersson, Q. Hu, M. Kritikos, T. Rasmussen and S. Ott, J. Org. Chem., 2009, 74, 9265–9273 CrossRef PubMed; (c) B. Schafer, E. Oberg, M. Kritikoz and S. Ott, Angew. Chem., Int. Ed., 2008, 47, 8228–8231 CrossRef PubMed; (d) V. A. Wright, B. O. Patrick, C. Schneider and D. P. Gates, J. Am. Chem. Soc., 2006, 126, 8836–8844 CrossRef PubMed; (e) C.-W. Tsang, B. Baharloo, D. Riendi, M. Yam and D. P. Gates, Angew. Chem., Int. Ed., 2004, 43, 5682–5685 CrossRef CAS PubMed; (f) C.-W. Tsang, M. Yam and D. P. Gates, J. Am. Chem. Soc., 2003, 125, 1480–1481 CrossRef CAS PubMed.
- (a) X. He, J. Borau-Garcia, A. Y. Y. Woo, S. Trudel and T. Baumgartner, J. Am. Chem. Soc., 2013, 135, 1137–1147 CrossRef CAS PubMed; (b) Y. Ren and T. Baumgartner, Dalton Trans., 2012, 41, 7792–7800 RSC; (c) P.-A. Bouit, A. Escande, R. Szucs, D. Szieberth, C. Lescop, L. Nyulaszi, M. Hissler and R. Reau, J. Am. Chem. Soc., 2012, 134, 6524–6527 CrossRef CAS PubMed; (d) A. Bruch, A. Fukazawa, E. Yamaguchi, S. Yamaguchi and A. Studer, Angew. Chem., Int. Ed., 2011, 50, 12094–12098 CrossRef CAS PubMed.
- (a) C. Mueller, R. Bartsch, A. Fischer, P. G. Jones and R. Schmutzler, J. Organomet. Chem., 1996, 512, 141–148 CrossRef CAS; (b) R. Appel, G. Maier, H. P. Reisenauer and A. Westerhaus, Angew. Chem., Int. Ed. Engl., 1981, 20, 197–197 CrossRef.
- (a) C. Jones and M. Waugh, J. Organomet. Chem., 2007, 692, 5086–5090 CrossRef CAS; (b) K. Toyota, S. Kawasaki and M. Yoshifuji, J. Org. Chem., 2004, 69, 5065–5070 CrossRef CAS PubMed.
- (a) A. S. Ionkin, W. J. Marshall, B. M. Fish, A. A. Marchione, L. A. Howe, F. Davidson and C. N. McEwen, Eur. J. Inorg. Chem., 2008, 2386–2390 CrossRef CAS; (b) G. Markl and H. Sejpka, Tetrahedron Lett., 1986, 27, 171–174 CrossRef.
- M. Yoshifuji, H. Kawanami, Y. Kawai, K. Toyota, M. Yasunami, T. Niitsu and N. Inamoto, Chem. Lett., 1992, 21, 1053–1056 CrossRef.
- (a) T. A. Cooper, H. W. Kroto, J. F. Nixon and O. Ohashi, J. Chem Soc., Chem. Commun., 1980, 333–334 RSC; (b) H. W. Kroto, J. F. Nixon and K. Ohno, J. Mol. Spectrosc., 1981, 90, 512–516 CrossRef CAS; (c) M. Brönstrup, J. Gottfriedsen, I. Kretzchmar, S. J. Blanksby, H. Schwarz and H. Schumann, Phys. Chem. Chem. Phys., 2000, 2, 2245–2250 RSC; (d) L. A. Jones, E. P. F. Lee, P. Soldan and T. G. Wright, Phys. Chem. Chem. Phys., 1999, 1, 391–395 RSC; (e) F. M. Bickelhaupt and F. Bickelhaupt, Chem. – Eur. J., 1999, 5, 162–174 CrossRef CAS.
- (a) F. Brodkorb, M. Brym, C. Jones and C. Schulten, J. Organomet. Chem., 2006, 691, 1025–1029 CrossRef CAS; (b) M. Brym and C. Jones, Dalton Trans., 2003, 3665–3667 RSC.
- P. B. Hitchcock, M. J. Maah, J. F. Nixon, J. A. Zora, G. J. Leigh and M. A. Bakar, Angew. Chem., Int. Ed. Engl., 1987, 26, 474–475 CrossRef.
- S. M. Mansell, M. Green and C. A. Russell, Dalton Trans., 2012, 41, 14360–14368 RSC.
- N. Trathen, M. C. Leech, I. R. Crossley, V. K. Greenacre and S. M. Roe, Dalton Trans., 2014, 43, 9004–9007 RSC.
- CCDC 1811689† Crystals grown from CH2Cl2/hexane at −20 °C. See ESI† for data.
- (a) M. C. B. Colber, J. Lewis, N. J. Long, P. R. Raithby, A. J. P. White and D. J. Williams, Dalton Trans., 1997, 99–104 RSC; (b) N. D. Jones and M. O. Wolf, Organometallics, 1997, 16, 1352–1354 CrossRef CAS; (c) C.-Y. Wong, C.-M. Che, M. C. W. Chan, J. Han, K.-H. Leung, D. L. Phillips, K.-Y. Wong and N. Zhu, J. Am. Chem. Soc., 2005, 127, 13997–14007 CrossRef CAS PubMed; (d) A. Vacher, F. Barriere, T. Roisnel, L. Piekara-Sady and D. Lorcy, Organometallics, 2011, 30, 3570–3578 CrossRef CAS.
- (a) S. Sporler, L. Muller, A. R. Waterloo, R. R. Tykwinski and N. Burzlaff, J. Organomet. Chem., 2016, 821, 122–129 CrossRef; (b) X. Wang, X. You, Z.-P. Shang and J. Xia, J. Organomet. Chem., 2016, 803, 111–118 CrossRef CAS; (c) J. Xia, Y.-P. Ou, D. Wu, G. J. Jin, J. Yin, G.-A. Yu and S. H. Liu, Dalton Trans., 2013, 42, 14212–14222 RSC; (d) M. A. Fox, B. Le Guennic, R. L. Roberts, D. A. Brue, D. S. Yufit, J. A. K. Howard, G. Manca, J.-F. Halet, F. Hartl and P. J. Low, J. Am. Chem. Soc., 2011, 133, 18433–18446 CrossRef CAS PubMed; (e) L. D. Field, A. M. Magill, T. K. Shearer, S. B. Colbran, S. T. Lee, S. J. Dalgarno and M. M. Bhadbhade, Organometallics, 2010, 29, 957–965 CrossRef CAS; (f) D. J. Armitt, M. I. Bruce, M. Gaudio, N. N. Zaitseva, B. W. Skelton, A. H. White, B. L. Guennic, J.-F. Halet, M. A. Fox, R. L. Roberts, F. Harlt and P. J. Low, Dalton Trans., 2008, 6763–6775 RSC.
- (a) J. G. Cordaro, D. Stein, H. Ruegger and H. Grutzmacher, Angew. Chem., Int. Ed., 2006, 45, 6159–6162 CrossRef CAS PubMed; (b) T. Groer, G. Baum and M. Scheer, Organometallics, 1998, 17, 5916–5919 CrossRef.
- Sub-stoichiometric KOtBu affords statistical mixture of 22+ and 3, with no evidence for the asymmetric (mono-desilylated) product. While separation of the mixture has not been effected, computed NMR data indicate signatures for the asymmetric species to be distinct from those of 22+ and 3.
- Geometries were optimized from an initial model based on the solid state structure of 22+, using the B3LYP functional (lanl2dz for ruthenium; 6-31G** for all other atoms). See ESI† for full details.
- C. Jones, C. Schulten and A. Stasch, Eur. J. Inorg. Chem., 2008, 1555–1558 CrossRef CAS.
- J. C. T. R. Burckett-St. Laurent, M. A. King, H. W. Kroto, J. F. Nixon and R. J. Suffolk, J. Chem. Soc., Dalton Trans., 1983, 755–759 RSC.
- TD-DFT calculations were performed at the B3LYP level using lanl2dz for Ru and 3-21G* for all other atoms, without addition of a solvent model. Though a relatively low level of theory (constituting a balance against the complexity of the system and number of required excited states), such has previously proven suitable to provide a general guide to assignment.
- Though commonly attributed to sequential RuII/RuIII couples, the oxidation events have heavy involvement from the carbon-rich bridge, due to extensive orbital mixing in the HOMO. These are thus more properly considered as sequential mono-oxidations of the bimetallic core.
- Though mindful of previous reports of 1 (and related systems) that describe the irreversible oxidation of [1]2+ close to 1 V,28b–e in the present case we are confident in our assignment of this feature to oxidation of the mixed-valence complex [22+]+ to [22+]2+, the initial event being more consistent with a 1-electron process.
- (a) E. Wuttke, Y.-M. Hervault, W. Polit, M. Linseis, P. Erler, S. Rigaut and R. F. Winter, Organometallics, 2014, 33, 4672–4686 CrossRef CAS; (b) A. Benameur, P. Brignou, E. D. Piazza, Y.-M. Hervault, L. Norel and S. Rigaut, New J. Chem., 2011, 35, 2105–2113 RSC; (c) C. Olivier, B. Kim, D. Touchard and S. Rigaut, Organometallics, 2008, 27, 509–518 CrossRef CAS; (d) A. Klein, O. Lavastre and J. Fielder, Organometallics, 2006, 25, 635–643 CrossRef CAS; (e) M. C. B. Colbert, J. Lewis, N. J. Long, P. R. Raithby, M. Younus, A. J. P. White, D. J. Williams, N. N. Payne, L. Yellowlees, D. Beljonne, N. Chawdhury and R. H. Friend, Organometallics, 1998, 17, 3034–3043 CrossRef CAS; (f) S. K. Hurst, M. P. Cifuentes, A. M. McDonagh, M. G. Humphrey, M. Samoc, B. Luther-Davies, I. Asselberghs and A. Persoons, J. Organomet. Chem., 2002, 642, 250–267 CrossRef; (g) O. Lavastre, J. Plass, P. Bachmann, S. Guesmi, C. Moinet and P. H. Dixneuf, Organometallics, 1997, 16, 184–189 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterising data and spectra, computational and electrochemical details, orbital plots, X-ray diffraction data. CCDC 1811689. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8dt00110c |
| This journal is © The Royal Society of Chemistry 2018 |