Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorescent antitumor titanium(IV) salen complexes for cell imaging†
Avia
Tzubery
a,
Naomi
Melamed-Book
b and
Edit Y.
Tshuva
 *a
*a
aInstitute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel. E-mail: edit.tshuva@mail.huji.ac.il
bThe Bio-Imaging Unit, The Alexander Silberman Institute of Life Sciences, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel
First published on 24th January 2018
Abstract
Two differently substituted fluorescent salen Ti(IV) complexes were developed. One was inactive on human cancer cells, whereas the other showed high cytotoxicity. Based on live cell imaging, both complexes penetrated the cell, but were not detected in the nuclei. Moreover, the inactive complex was trapped in endocytic vesicles, whereas the active complex accumulated in the perinuclear region and inflected phototoxicity upon continuous irradiation.
Titanium(IV) complexes are highly attractive candidates for anticancer therapy.1 After the platinum anticancer compounds, titanium(IV) complexes, in particular titanocene dichloride (Cp2TiCl2) and budotitane ((bzac)2Ti(OEt)2), were the first to enter clinical trials2 due to their high and wide activity in vitro and in vivo, accompanied by low toxicity, ultimately producing the non-toxic titanium dioxide in aqueous solutions.1–3 Nevertheless, these Ti(IV) compounds failed clinical trials due to their low hydrolytic stability in biological environment that led to the formation of undefined aggregates with low solubility, which also hampered mechanistic investigations.
Previously, we introduced anticancer Ti(IV) complexes based on phenolato ligands, which presented exceptional hydrolytic stability combined with high cytotoxicity and in vivo activity.1b,c,4 In particular, Ti(IV) LTiX2 type complexes based on diaminobis(phenolato) salan and salen ligands with two labile alkoxo groups were investigated, exhibiting activity often greater than that of cisplatin with slow hydrolysis to eventually yield highly stable and defined phenolato polynuclear clusters.4e,g,5 Despite the different geometries of the salan and salen complexes, similar mechanisms were suggested based on their similar reactivity with a bidentate chelating ligand and similar hydrolytic behavior.4g Structure–activity studies indicated strong impact of the ligand substitution on complex performance: steric bulk decreased activity, and hydrophobicity decreased solubility and accessibility and by that, affected reactivity.4d,5a,6
Very little is known about the mechanism of cytotoxicity of Ti(IV) complexes.1b,c Specifically, when analyzing potential cellular targets, various studies have been performed with older and newer compounds, the results of which are not all consistent. Although some studies pointed to possible interactions with DNA based on accumulation in nucleic acid-rich areas and various adducts identified, others suggested alternative mechanisms involving, for instance, enzyme inhibition.1c Herein, we utilize the photophysical features of the planar highly-conjugated salen system for the detection of Ti(IV) complexes in living cells using confocal fluorescence microscopy to shed light on the cellular bio-distribution of the cytotoxic phenolato Ti(IV) complexes. The π-conjugated system of the bis-Schiff base salen ligands can be modulated by particular substitutions on the amine bridge and/or the phenolato rings to enhance fluorescence.7 Such salen chelating ligands were previously used as optical probes for cations such as Cu(II), Pt(II), Al(III), and Zn(II),8 and salen Zn(II) complexes were employed as fluorescent markers for cell imaging.9
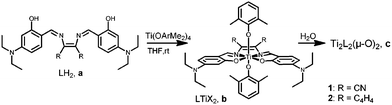
To enhance intramolecular charge transfer, diethylamino-substituted phenolato rings were employed, and two complexes were synthesized with cyano-substituted or phenylenediamine bridges (Scheme 1). The ligands 1a and 2a were prepared according to a known procedure via a condensation reaction between substituted salicylaldehyde and the conjugated diamine.7a The Ti(IV) complexes 1b and 2b were prepared as previously discussed for related compounds by reacting 1a and 2a, respectively, with one equiv. of titanium tetrakis(dimethylphenoxide) to obtain the Ti(IV) complexes in high yields.4f,g1H and 13C NMR analyses of the products confirmed the formation of a single isomer with high symmetry due to the single set of signals for the phenolato moiety of the salen ligand and a single type of a labile ligand. Single crystals of 1b and 2b were obtained from diethyl ether and dichloromethane, respectively, at −35 °C. Although the structure of 1b was of low quality (Fig. S1†), Fig. 1 depicts the ORTEP drawing of 2b along with selected bond lengths and angles.† Both structures clearly feature mononuclear octahedral C2v-symmetrical complexes with trans binding of the two labile dimethylphenoxo ligands. Furthermore, the bond lengths and angles of 2b around the metal center are similar to those of related Ti(IV) salen complexes.4f,g
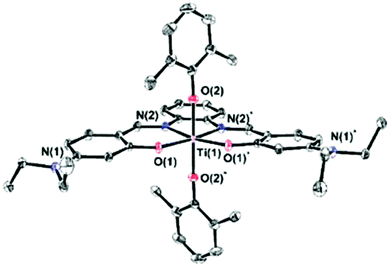 | ||
| Fig. 1 ORTEP drawing of 2b† with 50% probability ellipsoids; H atoms and dichloromethane solvent molecule were omitted for clarity. Selected bond lengths [Å] and angles [°]: Ti(1)–N(2) 2.143(2), Ti(1)–O(1) 1.904(2), Ti(1)–O(2) 1.874(2), O(1)–Ti(1)–O(2) 89.17(7), O(1)*–Ti(1)–O(1) 109.47(7), O(2)*–Ti(1)–O(2) 177.7(1), N(2)*–Ti(1)–N(2) 74.9(1), O(2)–Ti(1)–N(2) 90.25(7), and O(1)–Ti(1)–N(2) 87.88(7). | ||
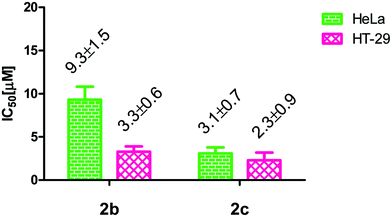
The cytotoxicity of 1b and 2b was evaluated on human colon HT-29 and cervix HeLa cancer cell lines using the methylthiazolyldiphenyl-tetrazolium (MTT) assay to establish cell viability (Fig. 2).10 Complex 2b exhibited high antitumor activity with IC50 values ranging from 3.3 to 9.3 μM, which are among the lowest values obtained for this family.4f,g In contrast, 1b was inactive toward both cell lines tested. It was suspected that the electron withdrawing cyano groups in 1b reduce the stability or affect cellular penetration due to reduced lipophilicity. The free salen ligand 2a analyzed as the control showed significantly lower reactivity (Fig. S7†).
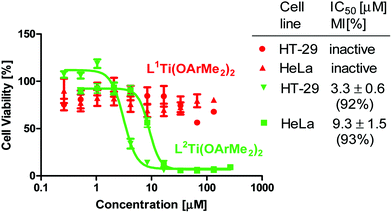 | ||
| Fig. 2 Dependence of HT-29 and HeLa cell viability (based on MTT assay after 3 days incubation) on concentration of 1b and 2b, which is presented on a logarithmic scale. | ||
The comparative hydrolytic stability of 1b and 2b was evaluated upon the addition of 10% D2O (ca. 1000 equiv. relative to Ti) to d6-DMSO solutions of the complexes, as previously described for related Ti(IV) compounds (Fig. S2†).4d,g,11 Spectra were collected every few minutes and the integration of selected signals of the labile 2,6-dimethylphenoxo ligands was measured relative to an internal standard. The hydrolytic stability of both complexes was similar with the t1/2 values for the labile ligand hydrolysis of 50 and 20 minutes for 1b and 2b respectively, ruling out reduced hydrolytic stability as the reason for the inactivity of 1b. Throughout the hydrolysis, single polynuclear species were separately obtained for each complex with no indication of salen ligand release even after 3 days. These products featured high symmetry, which indicated formation of either the di- or the tetra-nuclear product, as reported recently for related complexes,4g,11 whereby MS analysis supported the specific formation of a dimeric structure as characteristic for the salen family of complexes (Fig. S3†).4g The spectra of the hydrolysis products did not change even upon the addition of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 H2O equivalents to the parent complexes and stirring for 3 days, confirming the formation of a single defined cluster (Fig. S2c†).
000 H2O equivalents to the parent complexes and stirring for 3 days, confirming the formation of a single defined cluster (Fig. S2c†).
The hydrolytic behaviour of the complexes implied that the active species derived from 2b is its defined hydrolysis product.4e,12 Thus, to evaluate the possible reactivity of both hydrolysis products, complexes 1b and 2b were separately reacted with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 equivalents of H2O and the hydrolysis products 1c and 2c (Scheme 1) were isolated (Fig. S4 and S2c†). Their cytotoxicity was measured on HT-29 and HeLa cells, as described above. Although the hydrolysis product 1c was inactive like its parent complex, 2c exhibited high cytotoxicity with IC50 values comparable to those of its monomeric precursor and even better for the HeLa cell line (Fig. 3). Previous reports presented the cytotoxic activities of such defined polynuclear hydrolysis products based on salan ligands but only in formulations to increase solubility and accessibility12a or toward more sensitive murine cancer cell lines.12b Thus, this first observation of high activity of a defined phenolato cluster toward a relatively resistant human cancer cell line indicates the enhanced solubility and accessibility of 2c, and supports the overall conclusion that the hydrolysis product is the active species in the cell.
000 equivalents of H2O and the hydrolysis products 1c and 2c (Scheme 1) were isolated (Fig. S4 and S2c†). Their cytotoxicity was measured on HT-29 and HeLa cells, as described above. Although the hydrolysis product 1c was inactive like its parent complex, 2c exhibited high cytotoxicity with IC50 values comparable to those of its monomeric precursor and even better for the HeLa cell line (Fig. 3). Previous reports presented the cytotoxic activities of such defined polynuclear hydrolysis products based on salan ligands but only in formulations to increase solubility and accessibility12a or toward more sensitive murine cancer cell lines.12b Thus, this first observation of high activity of a defined phenolato cluster toward a relatively resistant human cancer cell line indicates the enhanced solubility and accessibility of 2c, and supports the overall conclusion that the hydrolysis product is the active species in the cell.
Studies with fluorescent Ti(IV) complexes are scarce13 because fluorescence quenching often occurs due to the electron-poor d0 Ti(IV) metal, which involves strong ligand to metal charge transfer.13 Nevertheless, 1b and 2b are highly emissive in DMSO with red emission for 1b and green-yellow emission for 2b, with similar features for their hydrolysis products 1c and 2c, respectively (Fig. S5, S6 and Table S1†). Additionally, having two related complexes in hand, one cytotoxic and one inactive, provided an opportunity to compare the two with respect to cellular bio-distribution. Thus, viable cells treated with 1b and 2b were evaluated using confocal fluorescence microscopy. The treatment of human cervical HeLa cells separately with 5 μM of 1b and 2b for only a few minutes resulted in the development of a strong red emission under excitation at 561 nm for 1b and green emission under excitation at 488 nm for 2b in the cytoplasm (Fig. 4). Interestingly, despite being inactive, 1b crossed the cell membrane relatively rapidly and also accumulated in the cytoplasm; therefore, its inactivity apparently does not result from its inability to enter the cell due to insufficient solubility/lipophilicity. Additionally, the defined hydrolysis products 1c and 2c were similarly analyzed at a concentration of 2 μM, and similar results and accumulation patterns were observed to those obtained for the parent complexes 1b and 2b, respectively.
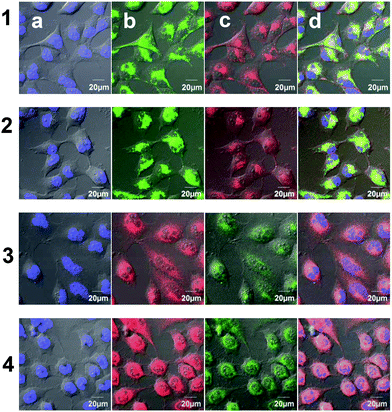
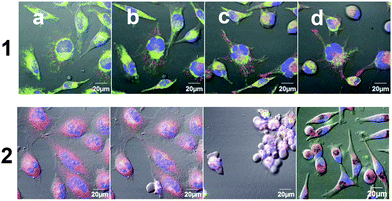
The subcellular accumulation of 1b and 2b and 1c and 2c was further investigated by staining the organelles with specific fluorescence probes: Hoechst® for staining the nuclei blue and MitoTracker® green or deep red for staining the mitochondria (applied for 1 or 2, respectively). Interestingly, no significant colocalization was detected for the Ti(IV) complexes, and in particular, no indication of entering the nuclei was observed.13 For the inactive 1b and its hydrolysis product 1c the signals appeared as spots and could be mapped as vesicles in the cytoplasm of the treated cells (Fig. 5, lines 1 and 2, respectively). These vesicles spread in the cell cytoplasm and their concentration increased with incubation time. This form of accumulation suggests that 1b/1c enter the cells via endocytosis in the form of endocytic vesicles and are transported towards the cytoplasm, as observed previously for Ru(II) complexes.14 Interestingly, a different process was observed for the cytotoxic 2b and its hydrolysis product 2c. These complexes crossed the membrane rapidly, possibly by diffusion, and accumulated in the perinuclear region, which could be associated with the endoplasmic reticulum (ER) domain or the Golgi apparatus, as presented in Fig. 5, lines 3 and 4, respectively. Some correlation was observed with MitoTracker®, which may result from mitochondria swelling. Thus, treatment with cytotoxic Ti(IV) compounds could induce mitochondrial dysfunction which eventually leads to apoptotic cell death. Interestingly, a previous study with Pt(II) complexes also showed accumulation in the ER region and induction of ER-stress and cell apoptosis,15 unlike the common observations of the nuclei as the target of Pt(II) complexes.16 Notably, the free ligand 2a, which was analyzed as the control, accumulated differently as defined spots near the nuclei (Fig. S8†).
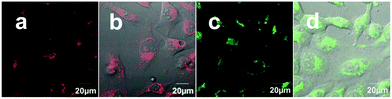
Following the addition of 5 μM of 2b to HeLa cells, as described above, and incubation under continuous irradiation (every 5–7 minutes), the viable cell population dramatically decreased, much more rapidly than expected (Fig. 6). After only 3 hours the cells underwent cell death and displayed cell shrinkage, loss of cell contact, and membrane blebbing (Fig. 2c, line 6), which are characteristic of early apoptosis.17 Despite the high cytotoxicity of 2b, such rapid cell death is not expected under the mild conditions employed. Interestingly, when different areas in the cell plate that did not undergo irradiation were measured, the majority of cells remained viable as expected (Fig. 6, line 2d). In contrast, for the inactive 1b, no indication of enhanced cell death was detected under irradiation, even after 16 hours of incubation under these conditions (Fig. 6, line 1). Thus, it is obvious that irradiation speeds up cell death, but only for the cytotoxic compound with a markedly lower effect for the control ligand 2a (Fig. S9†). Such phototoxicity was also observed for a cytotoxic Pt(II) complex of the salen ligand 2a (2a-Pt: Scheme S1 and Fig. S10†), which was analyzed as the control, showing accelerated cell death upon irradiation presumably due to its characteristic electronic properties.15
To conclude, we introduced herein the first fluorescent cytotoxic Ti(IV) complexes based on salen ligands bound directly to the metal that can be detected in live cells by confocal fluorescence microscopy. The cell imaging methodology offered new explanations for the factors limiting activity, which could not have been deduced based on the in vitro studies alone. Both the active and inactive complexes crossed the cell membrane and penetrated rapidly into the cytoplasm, which demonstrated that cell penetration is not a limiting factor for any of the compounds tested, despite their different lipophilicities. Nevertheless, the differently substituted inactive 1b seemed to accumulate in vesicles, which could have prevented its cytotoxicity by disabling its access to the biological target, possibly leading to decomposition in the lysosomes as the last stage of the endocytic pathway. In contrast, unlike the mildly active free ligand 2a, the cytotoxic 2b and its suggested active species 2c localized in the perinuclear region. These complexes could also be further light-activated for significantly increased cytotoxic activity due to an associated phototoxicity process, which was also previously encountered with Pt(II) and Ru(II) complexes.15,18 This provides an additional possible advantage of the salen Ti(IV) complexes for application in controlled anticancer therapy. Further studies with additional detection techniques are required to unequivocally determine the biological target of these promising anticancer Ti(IV) complexes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr B Bogoslavsky for X-ray analysis. Funding was received from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement 681243).Notes and references
- (a) E. Meléndez, Crit. Rev. Oncol. Hematol., 2002, 42, 309 CrossRef; (b) S. A. Loza-Rosas, M. Saxena, Y. Delgado, K. Gaur, M. Pandrala and A. D. Tinoco, Metallomics, 2017, 9, 346 RSC; (c) M. Cini, T. D. Bradshaw and S. Woodward, Chem. Soc. Rev., 2017, 46, 1040 RSC; (d) E. Y. Tshuva and M. Miller, in Metallo-Drugs: Development and Action of Anticancer Agents, Walter de Gruyter, Berlin, 2018, vol. 18, pp. 219–249 Search PubMed; (e) K. M. Buettner and A. M. Valentine, Chem. Rev., 2012, 112, 1863 CrossRef CAS PubMed; (f) W. A. Wani, S. Prashar, S. Shreaz and S. Gomez-Ruiz, Coord. Chem. Rev., 2016, 312, 67 CrossRef CAS.
- (a) P. Köpf-Maier and H. Köpf, Chem. Rev., 1987, 87, 1137 CrossRef; (b) G. Lümmen, H. Sperling, H. Luboldt, T. Otto and H. Rubben, CANCER Chemother. Pharmacol., 1998, 42, 415 CrossRef PubMed; (c) T. Schilling, K. B. Keppler, M. E. Heim, G. Niebch, H. Dietzfelbinger, J. Rastetter and A. R. Hanauske, Invest. New Drugs, 1995, 13, 327 CrossRef CAS; (d) B. K. Keppler, C. Friesen, H. G. Moritz, H. Vongerichten and E. Vogel, Struct. Bonding, 1991, 78, 97 CrossRef CAS; (e) F. Caruso, M. Rossi and C. Pettinari, Expert Opin. Ther. Pat., 2001, 11, 969 CrossRef CAS; (f) N. Kröger, U. R. Kleeberg, K. Mross, L. Edler and D. K. Hossfeld, Oncol. Res. Treat., 2000, 23, 60 CrossRef.
- (a) F. Caruso, L. Massa, A. Gindulyte, C. Pettinari, F. Marchetti, R. Pettinari, M. Ricciutelli, J. Costamagna, J. C. Canales, J. Tanski and M. Rossi, Eur. J. Inorg. Chem., 2003, 3221 CrossRef CAS; (b) I. Kostova, Anticancer Agents Med. Chem., 2009, 9, 827 CrossRef CAS PubMed; (c) K. Strohfeldt and M. Tacke, Chem. Soc. Rev., 2008, 37, 1174 RSC; (d) J. H. Toney and T. J. Marks, J. Am. Chem. Soc., 1985, 107, 947 CrossRef CAS; (e) P. Köpf-Maier and H. Köpf, Struct. Bonding, 1988, 70, 103 CrossRef; (f) A. Sigel and H. Sigel, Metal complexes in tumor diagnosis and as anticancer agents, Marcel Dekker, Inc., New York, 2004 Search PubMed; (g) F. Caruso and M. Rossi, Mini-Rev. Med. Chem., 2004, 4, 49 CrossRef CAS PubMed.
- (a) M. Shavit, D. Peri, C. M. Manna, J. S. Alexander and E. Y. Tshuva, J. Am. Chem. Soc., 2007, 129, 12098 CrossRef CAS PubMed; (b) E. Y. Tshuva and D. Peri, Coord. Chem. Rev., 2009, 253, 2098 CrossRef CAS; (c) E. Y. Tshuva and J. A. Ashenhurst, Eur. J. Inorg. Chem., 2009, 2203 CrossRef CAS; (d) D. Peri, S. Meker, M. Shavit and E. Y. Tshuva, Chem. – Eur. J., 2009, 15, 2403 CrossRef CAS PubMed; (e) D. Peri, S. Meker, C. M. Manna and E. Y. Tshuva, Inorg. Chem., 2011, 50, 1030 CrossRef CAS PubMed; (f) A. Tzubery and E. Y. Tshuva, Inorg. Chem., 2011, 50, 7946 CrossRef CAS PubMed; (g) A. Tzubery and E. Y. Tshuva, Inorg. Chem., 2012, 51, 1796 CrossRef CAS PubMed; (h) M. Miller, O. Braitbard, J. Hochman and E. Y. Tshuva, J. Inorg. Biochem., 2016, 163, 250 CrossRef CAS PubMed.
- (a) T. A. Immel, U. Groth and T. Huhn, Chem. – Eur. J., 2010, 16, 2775 CrossRef CAS PubMed; (b) T. A. Immel, M. Debiak, U. Groth, A. Bürkle and T. Huhn, ChemMedChem, 2009, 4, 738 CrossRef CAS PubMed; (c) T. A. Immel, U. Groth, T. Huhn and P. Öhlschläger, PLoS One, 2011, 6, 1 Search PubMed.
- (a) H. Glasner and E. Y. Tshuva, J. Am. Chem. Soc., 2011, 133, 16812 CrossRef CAS PubMed; (b) H. Glasner and E. Y. Tshuva, Inorg. Chem., 2014, 53, 3170 CrossRef CAS PubMed; (c) M. Miller and E. Y. Tshuva, Eur. J. Inorg. Chem., 2014, 1485 CrossRef CAS.
- (a) J. Cheng, K. Wei, X. Ma, X. Zhou and H. Xiang, J. Phys. Chem. C, 2013, 117, 16552 CrossRef CAS; (b) C. J. Whiteoak, G. Salassa and A. W. Kleij, Chem. Soc. Rev., 2012, 41, 622 RSC.
- Repesentative examples: (a) L. Zhou, P. Cai, Y. Feng, J. Cheng, H. Xiang, J. Liu, D. Wu and X. Zhou, Anal. Chim. Acta, 2012, 735, 96 CrossRef CAS PubMed; (b) L. Zhou, Y. Feng, J. Cheng, N. Sun, X. Zhou and H. Xiang, RSC Adv., 2012, 2, 10529 RSC; (c) S. Samanta, B. Nath and J. B. Baruah, Inorg. Chem. Commun., 2012, 22, 98 CrossRef CAS; (d) Y. Zhou, H. N. Kim and J. Yoon, Bioorg. Med. Chem. Lett., 2010, 20, 125 CrossRef CAS PubMed.
- (a) D. Xie, J. Jing, Y.-B. Cai, J. Tang, J.-J. Chen and J.-L. Zhang, Chem. Sci., 2014, 5, 2318 RSC; (b) Y. Hai, J.-J. Chen, P. Zhao, H. Lv, Y. Yu, P. Xu and J.-L. Zhang, Chem. Commun., 2011, 47, 2435 RSC.
- N. Ganot, S. Meker, L. Reytman, A. Tzubery and E. Y. Tshuva, J. Visualized Exp., 2013, e50767 Search PubMed.
- A. Tzubery and E. Y. Tshuva, Eur. J. Inorg. Chem., 2017, 1695 CrossRef CAS.
- (a) S. Meker, K. Margulis-Goshen, E. Weiss, S. Magdassi and E. Y. Tshuva, Angew. Chem., Int. Ed., 2012, 51, 10515 CrossRef CAS PubMed; (b) S. Meker, K. Margulis-Goshen, E. Weiss, O. Braitbard, J. Hochman, S. Magdassi and E. Y. Tshuva, ChemMedChem, 2014, 9, 1294 CrossRef CAS PubMed.
- (a) G. Khalil, C. Orvain, L. Fang, L. Barloy, A. Chaumont, C. Gaiddon, M. Henry, N. Kyritsakas and P. Mobian, Dalton Trans., 2016, 45, 19072 RSC; (b) O. Florès, A. Trommenschlager, S. Amor, F. Marques, F. Silva, L. Gano, F. Denat, M. P. Cabral Campello, C. Goze, E. Bodio and P. Le Gendre, Dalton Trans., 2017, 46, 14548 RSC.
- W.-K. Tsui, L.-H. Chung, M. M.-K. Wong, W.-H. Tsang, H.-S. Lo, Y. Liu, C.-H. Leung, D.-L. Ma, S.-K. Chiu and C.-Y. Wong, Sci. Rep., 2015, 5, 9070 CrossRef CAS PubMed.
- T. Zou, C.-N. Lok, Y. M. E. Fung and C.-M. Che, Chem. Commun., 2013, 49, 5423 RSC.
- (a) J. Gao, Y.-G. Liu and R. A. Zingaro, Chem. Res. Toxicol., 2009, 22, 1705 CrossRef CAS PubMed; (b) N. Kumari, B. K. Maurya, R. K. Koiri, S. K. Trigun, S. Saripella, M. P. Coogan and L. Mishra, MedChemComm, 2011, 2, 1208 RSC.
- V. H. S. van Rixel, B. Siewert, S. L. Hopkins, S. H. C. Askes, A. Busemann, M. A. Siegler and S. Bonnet, Chem. Sci., 2016, 7, 4922 RSC.
- J. Hess, H. Huang, A. Kaiser, V. Pierroz, O. Blacque, H. Chao and G. Gasser, Chem. – Eur. J., 2017, 23, 9888 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedures, spectroscopic data and crystal data of 2b. CCDC 1584442. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt04828a |
| This journal is © The Royal Society of Chemistry 2018 |