Open Access Article
Open Access ArticleCrystal structures of uranyl complexes with isobutyrate and isovalerate anions†
Anton V.
Savchenkov
 *a,
Anna V.
Vologzhanina
*a,
Anna V.
Vologzhanina
 b,
Artem O.
Dmitrienko
b,
Artem O.
Dmitrienko
 b,
Yan V.
Zubavichus
b,
Yan V.
Zubavichus
 c,
Denis V.
Pushkin
c,
Denis V.
Pushkin
 a,
Larisa B.
Serezhkina
a and
Viktor N.
Serezhkin
a
a,
Larisa B.
Serezhkina
a and
Viktor N.
Serezhkin
a
aSamara National Research University, 443011 Samara, Russian Federation. E-mail: anton.savchenkov@gmail.com
bNesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, 119991 Moscow, Russian Federation
cNational Research Center “Kurchatov Institute”, 123182 Moscow, Russian Federation
First published on 16th January 2018
Abstract
Single crystals of Na[(UO2)(i-C3H7COO)3]·0.7H2O (I), Cs[(UO2)(i-C3H7COO)3] (II) and (NH4)[(UO2)(i-C4H9COO)3] (III) were obtained via isothermal evaporation and their structures were solved using X-ray diffraction techniques. Even though the ligands are branched, bulky and spatial, many carbon and hydrogen atoms are still disordered in these crystal structures at low temperature. A new type of Na coordination is observed for the first time for this family of compounds, proposing high sensitivity of compound I to humidity. Depolymerization of the metal–oxygen frameworks for the new compounds is compared with the known ones. Coordination sequences of sodium/cesium and uranyl complexes with aliphatic monocarboxylate ions are calculated to show different crystal-chemical function of crystallographically independent atoms. As there are analogous compounds to the title ones with straight-chain ligands, such groups of similar compounds with single varying parameters are very advantageous for establishing correlations between composition and crystal structure.
Introduction
Many scientific groups are extensively studying different aspects of uranium chemistry due to its high potential for the nuclear industry (for example, ref. 1–5). A lot of attention is devoted to carboxylate complexes of uranyl ions since they are loosely related to the nuclear fuel cycle, they occur in nature, they are used for staining in electron microscopy, etc.6–9 Earlier, our continuous efforts on the synthesis of coordination compounds of uranyl ions with higher homologues of acetate ions resulted in several new butyrate and valerate containing compounds (for example, ref. 10–12). As known, in most cases crystallization of uranyl ions and anions of monocarboxylic acids results in the formation of mononuclear anionic complex groups [UO2L3]−, where L is a monocarboxylate ion.13 The monocarboxylate ions L in such building units realize a bidentate cyclic coordination mode, which is designated as B01 in accordance with the nomenclature in ref. 14. With a few exceptions, the vast majority of structures of uranyl complexes with aliphatic monocarboxylate ions are three-dimensional due to electrostatic interactions among complex anions [UO2L3]− and counterions.13The common feature of almost all known uranyl compounds with butyrate and valerate ions is the severe disorder of the hydrocarbon chains due to their high flexibility. The aim of the current work was to synthesize uranyl complexes with branched isobutyrate and isovalerate ions with the assumption that such ligands would be ordered in crystal structures as they allow more tight packing. As a result of our synthetic efforts we succeeded in growing single crystals and solving structures of Na[(UO2)(i-C3H7COO)3]·0.7H2O (I), Cs[(UO2)(i-C3H7COO)3] (II) and (NH4)[(UO2)(i-C4H9COO)3] (III). Even though compounds I–III contain bulky spatial branched ligands, many carbon and hydrogen atoms are still disordered in these crystal structures. The derived compounds I and II are analogous to the previously reported complexes Na[(UO2)(n-C3H7COO)3]·0.25H2O,15 Cs[UO2(n-C3H7COO)3]16 and Cs[UO2(n-C3H7COO)3][UO2(n-C3H7COO)(OH)(H2O)]17 with straight-chain ligands. With the absence of the known straight-chain valerate complex of uranyl and ammonium ions, the closest analogs to compound III are the only reported up to date isovalerate containing uranyl complex Na4(UO2)4(i-C4H9COO)11(NO3)·3H2O18 and two polymorphs of the crotonate complex of uranyl and ammonium ions, NH4[UO2(C3H5COO)3].19
Although all the mentioned compounds seem quite similar, it was shown earlier that their structures may feature different peculiarities, such as topological isomerism,10 depolymerization of the metal–oxygen frameworks,18 different packings with different systems of noncovalent interactions,19 and relation between the crystal system of compounds and the volumes of Voronoi–Dirichlet polyhedra of cations and ligands.16,20 Finally, such groups of similar compounds with single varying parameters (like the type of the ligand: branched or straight, or the cation) are very advantageous for establishing correlations between composition and crystal structure. Such correlations as well as structural peculiarities of the mentioned compounds are discussed below. Thus, besides having a possible practical application in the nuclear industry, the newly synthesized compounds serve in the development of theoretical crystal chemistry.
Experimental section
Syntheses
Caution! Compounds of U represent a potential health risk owing to radioactivity. Although the uranium precursors used contain depleted uranium, standard safety measures for handling radioactive substances must be followed.Single crystals of I–III were obtained via isothermal evaporation of solutions in 20 or 100 ml glass vials at room temperature. Uranium oxide (UO3) was prepared according to the earlier reported procedure.21 Isobutyric acid (i-C3H7COOH, 99%), isovaleric acid (i-C4H9COOH, 99%), sodium hydroxide (NaOH, reagent grade, ≥98%), cesium hydroxide monohydrate (CsOH·H2O, technical grade) and ammonium hydroxide solution (ACS reagent, 28.0–30.0% NH3 in H2O) were obtained commercially from Sigma-Aldrich.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Evaporation of the transparent yellow solution in a few days resulted in yellow platy crystals that appeared to be the known phase, [UO2(i-C3H7COO)2(H2O)2].22 After a few more days of evaporation yellow prismatic crystals formed that appeared to be the new phase, Na[(UO2)(i-C3H7COO)3]·0.7H2O (I). The yield of the second phase was: ≈15%. Gravimetric analysis of uranium content in I resulted in a value of 41.9% (calculated 42.0%).
1, respectively. Evaporation of the transparent yellow solution in a few days resulted in yellow platy crystals that appeared to be the known phase, [UO2(i-C3H7COO)2(H2O)2].22 After a few more days of evaporation yellow prismatic crystals formed that appeared to be the new phase, Na[(UO2)(i-C3H7COO)3]·0.7H2O (I). The yield of the second phase was: ≈15%. Gravimetric analysis of uranium content in I resulted in a value of 41.9% (calculated 42.0%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Evaporation of the transparent yellow solution in a few days resulted in yellow prismatic crystals that appeared to be the new phase, Cs[(UO2)(i-C3H7COO)3] (II). The yield was: ≈65%. Gravimetric analysis of uranium content in II resulted in a value of 35.7% (calculated 35.8%).
1, respectively. Evaporation of the transparent yellow solution in a few days resulted in yellow prismatic crystals that appeared to be the new phase, Cs[(UO2)(i-C3H7COO)3] (II). The yield was: ≈65%. Gravimetric analysis of uranium content in II resulted in a value of 35.7% (calculated 35.8%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. Evaporation of the transparent yellow solution in a few days resulted in a mixture of yellow platy and prismatic crystals. Platy crystals appeared to be the amorphous uranyl isovalerate, whose crystalline state has not been reported yet. Crystalline prismatic crystals appeared to be the new phase, (NH4)[(UO2)(i-C4H9COO)3] (III). The yield of compound III was: ≈10%. Gravimetric analysis of uranium content in III resulted in a value of 43.2% (calculated 43.4%).
2, respectively. Evaporation of the transparent yellow solution in a few days resulted in a mixture of yellow platy and prismatic crystals. Platy crystals appeared to be the amorphous uranyl isovalerate, whose crystalline state has not been reported yet. Crystalline prismatic crystals appeared to be the new phase, (NH4)[(UO2)(i-C4H9COO)3] (III). The yield of compound III was: ≈10%. Gravimetric analysis of uranium content in III resulted in a value of 43.2% (calculated 43.4%).
X-ray diffraction
Single crystals of I–III were obtained from reaction mixtures. The intensities of reflections for I and II were measured with a Bruker Apex II DUO CCD diffractometer using Mo-Kα radiation (λ = 0.71073) at 120.0(2) K and were merged by SADABS.23 Intensity data for III were collected at 100.0(2) K at the BELOK beamline of the Kurchatov Synchrotron Radiation Source (Moscow, Russia) at a wavelength of 0.9699 Å using a Rayonix SX-165 CCD detector and merged using the SCALA24 package. All structures were solved by the direct method and refined by full-matrix least squares against F2. Non-hydrogen atoms were refined anisotropically except for some disordered atoms. The disordered carbon atoms (some methyl groups in I and the single unique alkyl group in III) were refined isotropically. A number of EADP, ISOR, SADI, RIGU and DFIX instructions were applied to refine some moieties. TWIN/BASF refinement was performed for III. Positions of hydrogen atoms were calculated and included in the refinement by the riding model with Uiso(H) = 1.5Ueq(X) for methyl groups and water molecules, and Uiso(H) = 1.2Ueq(X) for other atoms. All calculations were made using the SHELXL2014![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and OLEX2
25 and OLEX2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 program packages.
26 program packages.
Unfortunately, although atomic coordinates of all atoms could be obtained for a single crystal of II, including carbon atoms of a disordered anion, for all tested single crystals we obtained high convergence factors (Rint and Rf were higher than 0.3 and 0.12, respectively) and poor Flack parameter (0.2), anomalous bond distances and thermal parameters. The quality of single crystals decreased at low temperatures indicating the presence of twinning, but we failed to detwin our data. So, further refinement of the atomic coordinates and thermal parameters of II was carried out at room temperature using powder XRD.
The powder pattern of II was measured on a Bruker D8 Advance Vario diffractometer at RT with a LynxEye detector and Ge (111) monochromator, λ(CuKα1) = 1.54060 Å, θ/2θ scan from 5.6° to 89.5°, step size 0.00786°. The measurement was performed in the transmission mode, with the sample deposited between two Kapton films. All calculations were performed with the Bruker TOPAS package.27 The pattern was indexed with the SVD method28 in a cubic crystal system with crystal parameters a = 12.39075(4) Å and V = 1902.360(11) Å3. The space group P213 (similar to that obtained for a single crystal of II) was evaluated based on systematic absences. The atomic coordinates taken from single-crystal solution were included in the Rietveld refinement, with restraints applied to all covalent and metal–oxygen bonds, bond angles and metal atoms fixed on three-fold rotation axes. Hydrogen atoms were refined by the riding model. For each type of non-hydrogen atoms an isotropic thermal parameter was refined. The model was refined with the restraints proposed in ref. 29 and the average half-uncertainty window for the refinement as 0.10(2), indicating a consistent structural model. The preferred orientation was described with 4th order spherical harmonics. Line asymmetry was refined in a full axial model.30 At an average Δd of 0.01 Å the refinement converged to Rwp/R′wp/Rp/R′p/RBragg = 2.96/6.67/2.15/7.15/1.15%.
Details of data collection and structure refinement parameters for I and III are given in Table 1. The atomic coordinates were deposited at the Cambridge Crystallographic Data Centre, CCDC no. 1579816–1579818† for I–III, respectively.31 The following figures with fragments of crystal structures were prepared using the VESTA 3 visualization system.32
| Compound | I | II | III |
|---|---|---|---|
| Empirical formula | Na[(UO2)(i-C3H7COO)3]·0.7H2O | Cs[(UO2)(i-C3H7COO)3] | (NH4)[(UO2)(i-C4H9COO)3] |
| Crystal system, space group, Z | Orthorhombic, P212121, 20 | Cubic, P213, 4 | Cubic, P213, 4 |
| a, Å | 21.051(4) | 12.39075(4) | 12.6458(12) |
| b, Å | 21.361(4) | 12.39075(4) | 12.6458(12) |
| c, Å | 21.402(4) | 12.39075(4) | 12.6458(12) |
| V, Å3 | 9624(3) | 1902.360(11) | 2022.3(6) |
| D x, g cm−3 | 1.956 | 2.318 | 1.943 |
| μ, mm−1 | 8.839 | 8.064 | |
| Crystal size, mm | 0.40 × 0.25 × 0.21 | 0.29 × 0.26 × 0.19 | |
| θ range, ° | 3.678–38.483 | 2.278–29.865 | |
| h, k, l range | −25 ≤ h ≤ 25, −26 ≤ k ≤ 26, −26 ≤ l ≤ 26 | −17 ≤ h ≤ 17, −17 ≤ k ≤ 17, −17 ≤ l ≤ 17 | |
| Reflections number: collected/unique (N1) | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705/20 705/20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732 732 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814/1934 814/1934 |
|
| R int/with I > 2σ(I) (N2) | 0.1457/14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 289 289 |
0.1682/1539 | |
| Parameters refined | 978 | 73 | |
| wR2 on N1 | 0.2000 | 0.1657 | |
| R 1 on N2 | 0.0805 | 0.0773 | |
| S | 1.146 | 1.019 | |
| Absolute structure parameter x33 | 0.110(6) | 0.24(6) | |
| Δρmax/Δρmin, e Å−3 | −2.925/4.514 | −2.791/2.915 |
FTIR spectroscopy
The FTIR spectra of compounds I–III were measured as pressed KBr pellets in the range of 500–3500 cm−1 using a PerkinElmer Spectrum 100 FTIR spectrometer. Assignment of absorption bands (provided in the ESI†) was carried out according to the published materials.34 Antisymmetric stretching vibrations of uranyl ions arise at 930 (I), 929 (II) and 928 (III) cm−1. A distinctive feature of the branched hydrocarbon chain of isocarboxylate ions is the Fermi doublet of δ(CH) bending vibrations at approximately 1370 and 1380 cm−1 with almost equal intensities.35Results and discussion
Crystals of I belong to the orthorhombic crystal system (space group P212121, Z′ = 5). Five crystallographic sorts of uranium and five of sodium atoms occupy general positions. The U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O distances and O
O distances and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O angles of the uranyl ions fall in the ranges of 1.66(3)–1.82(2) Å and 177.6(10)–179.0(15)°. Compounds II and III crystallize in the space group P213 (Z′ = 1/3), which is rare among all compounds in CSD and ICSD,31,36,37 but is frequently observed for other uranyl carboxylates.16,20 In II and III, Cs, N and U atoms occupy special positions with C3 site-symmetry, as well as O atoms of uranyl ions in both crystal structures and one out of four H atoms of the ammonium ion in III. Thus, the uranyl ions in II and III are linear with U
O angles of the uranyl ions fall in the ranges of 1.66(3)–1.82(2) Å and 177.6(10)–179.0(15)°. Compounds II and III crystallize in the space group P213 (Z′ = 1/3), which is rare among all compounds in CSD and ICSD,31,36,37 but is frequently observed for other uranyl carboxylates.16,20 In II and III, Cs, N and U atoms occupy special positions with C3 site-symmetry, as well as O atoms of uranyl ions in both crystal structures and one out of four H atoms of the ammonium ion in III. Thus, the uranyl ions in II and III are linear with U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O distances in the range of 1.75(2)–1.76(3) Å.
O distances in the range of 1.75(2)–1.76(3) Å.
Coordination polyhedra of all U atoms in I–III are hexagonal bipyramids (Fig. 1 and 2). The volumes of the Voronoi–Dirichlet polyhedra of U atoms in I–III are in the range of 9.08–9.50 Å3, which is in good agreement with the known value of 9.3(2) Å3 for U(VI) atoms in UOn polyhedra with n = 5, 6, 7, 8 or 9.38 In equatorial planes, the uranyl ions coordinate six oxygen atoms belonging to three isobutyrate or isovalerate ions with the B01 bidentate cyclic coordination mode (Fig. 1 and 2).14 All three crystals are constructed of typical anionic complex units [UO2L3]−, where L is an isobutyrate or isovalerate ion (Fig. 1 and 2). The crystallochemical formula14 of such complexes is AB013, where A = UO22+ and B01 = i-C3H7COO− or i-C4H9COO−. Many carbon and hydrogen atoms of the hydrocarbon chains in I–III are disordered over two positions. In I, one out of four independent water molecules has an occupancy of 0.5.
The anionic complex units [UO2L3]− in I–III are bonded into 3D framework structures through electrostatic interactions with Na or Cs atoms and through hydrogen bonds with ammonium ions. As the C3 axis goes through the N1 and H1B atoms of an ammonium ion, it forms symmetry related hydrogen bonds: three moderate hydrogen bonds N1–H1A⋯O3 and one weak trifurcated hydrogen bond N1–H1B⋯O4 (see Fig. 3 and Table 2).39,40 The hydrogen atoms of water molecules in I are also involved in moderate hydrogen bonding (Fig. 1, Table 2). The values of Ω(H⋯O) and Ω(A⋯O), which proved to be extremely valuable for the determination of hydrogen bonding even in the absence of coordinates of hydrogen atoms,41,42 also indicate moderate and weak hydrogen bonding in I and III (see Table 2). Full projections of crystal structures of I–III are provided in the ESI.†
| A–H⋯O | Distance, Å | Angle A–H⋯O, ° | Ω(H⋯O), % | Ω(A⋯O), % | ||
|---|---|---|---|---|---|---|
| A⋯O | A–H | H⋯O | ||||
| a Ω is the solid angle (in percent of 4π steradian), at which the shared face of the Voronoi–Dirichlet polyhedra of adjacent atoms is seen from the nucleus of any of them. | ||||||
| Na[(UO2)(i-C3H7COO)3]·0.7H2O (I) | ||||||
| O41–H41A⋯O38 | 2.87 | 0.85 | 2.02 | 177.5 | 19.2 | 14.8 |
| O41–H41B⋯O39 | 2.77 | 0.85 | 2.14 | 130.4 | 13.3 | 15.1 |
| O42–H42A⋯O17 | 3.19 | 0.85 | 2.35 | 174.3 | 15.2 | 11.1 |
| O42–H42B⋯O36 | 2.82 | 0.85 | 1.97 | 179.0 | 21.0 | 16.7 |
| O43–H43B⋯O5 | 2.47 | 0.86 | 2.07 | 108.3 | 15.6 | 17.9 |
| O44–H44A⋯O12 | 3.03 | 0.85 | 2.24 | 156.1 | 16.8 | 14.1 |
| O44–H44B⋯O13 | 2.84 | 0.85 | 2.06 | 152.7 | 18.5 | 15.3 |
| (NH4)[(UO2)(i-C4H9COO)3] (III) | ||||||
| N1–H1A⋯O3 | 2.86 | 0.97 | 1.90 | 172.7 | 23.2 | 17.1 |
| N1–H1B⋯O4 trifurcated | 2.93 | 0.82 | 2.52 | 111.9 | 9.1 | 13.9 |
As in the case of several earlier studied uranyl complexes,10,18 the three-dimensional frameworks of crystal structures of I and II have the NaUO6 or CsUO6 composition, in which U atoms with coordination number equal to 8 are interconnected with Na or Cs atoms via the equatorial O atoms (Fig. 4a). All the remaining atoms and groups of atoms are terminal and ‘hang’ on the mentioned framework (the hydrocarbon chains, the O atoms of the uranyl cations and the water molecules).
The coordination numbers (CN) of Na and Cs atoms were calculated using the method of intersecting spheres.43,44 This method gives a strict value of CN for any atom in any crystal lattice and allows overcoming the ambiguity of defining the CN for alkali metals.45 According to our calculations the single Cs atom in II has CN = 6. The bond distances within the CsO6 coordination polyhedron are in the range of 3.06–3.10 Å. The CsO6 polyhedron consists of oxygen atoms of six different isobutyrate ions belonging to three neighbouring [UO2(i-C3H7COO)3]− complexes, which is typical of the cubic crystals of uranyl complexes with ions of aliphatic monocarboxylic acids (for example, ref. 16 and 46–48). The resulting 3D framework is cubic and represents the optimal ratio between the size of the pores in the CsUO6 framework and the size of the isobutyrate ligands.10,16,20 It is worth noting, that the structure of compound II lacks the uranyl-cation UO22+–Cs+ interactions, which are frequently observed in other uranyl and cesium containing compounds,48–50 but also were not observed in the analogous compound Cs[UO2(n-C3H7COO)3] with unbranched n-butyrate ions.16
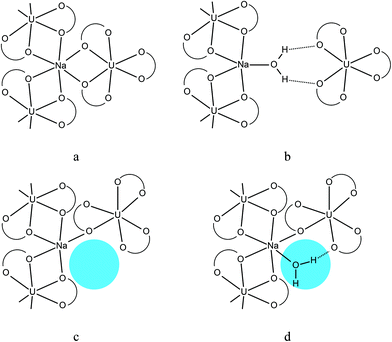
In ref. 18, which features the discussion of sodium containing carboxylate complexes of uranyl ions, two types of sodium ions were described. The first type of sodium atoms is characterized with CN 6 and chemical bonds with six oxygen atoms of three neighboring complex ions [UO2L3]− (Fig. 4a). In such a case, every U (Na) atom is connected to three Na (U) atoms through double –O– bridges (Fig. 4a). The Na atoms of the second type lose two chemical bonds with the O atoms of one of the neighboring complex ions, [UO2L3]−; instead they bind one water molecule, which in total decreases their CN to 5 (Fig. 4b). This effect leads to the partial depolymerization of the metal–oxygen NaUO6 framework of the corresponding structures and to the lowering of the values of coordination sequences, which show the number of Na or U atoms (CP) bonded via R–(O–R–)N chains, where R is either Na or U, in the Nth coordination sphere around the central atom.51
The CN's of Na atoms in compound I are also equal either to 5 (Na1, Na2, Na3, Na5) or to 6 (Na4), although their structural function appeared to be different. Atoms Na1, Na2 and Na5 are similar to Na atoms with CN 5 from the cited paper:18 they bind only two neighboring complex anions [UO2L3]− and one water molecule and they lead to the depolymerization of the framework (Fig. 4b). On the other hand, each of the Na3 and Na4 atoms is bonded with two neighboring complex anions [UO2L3]− through double Na–O–U bridges and with the third complex anion – through a single Na–O–U bridge (Fig. 4c and d). Moreover, the Na4 atom binds one water molecule (Fig. 4d). Such a coordination environment of Na3 and Na4 atoms saves the framework from depolymerization in those nodes, which is more like the situation with the ‘old’ potassium uranyl acetate, K[UO2(CH3COO)3]·0.5H2O,52 described in the cited paper.16 In the case of the Na3 atom the coordination polyhedron is very distorted and there is a void big enough for allocating one water molecule – similar to the case of the Na4 atom, which coordinates one water molecule with halved occupancy. That fact means that compound I should be very sensitive to humidity, as two out of five Na atoms can lose or gain one water molecule each without significant structural rearrangements, which is 0.4 water molecules per formula unit.
Such different coordination environments of Na atoms result in different coordination sequences for Na and for U atoms in crystal structures of the compounds under discussion.10,18,51Table 3 lists the coordination sequences CP for the five unique U atoms in the structure of compound I. While U1, U3 and U4 atoms have almost identical values of CP, the corresponding values for U2 and U5 are significantly lower. In fact, the U5 atom has the least number of neighboring atoms in the first three coordination spheres among all the known uranyl complexes with aliphatic monocarboxylate ions (Fig. 5). Thus, in spite of the similar coordination polyhedra of all five unique U atoms in the crystal structure of I, their structural function is very different in further coordination spheres. Although all the known sodium and uranyl complexes with aliphatic monocarboxylate ions feature the same composition of the metal–organic frameworks, NaUO6, their structures are different, which is an another example of topological isomerism.
| Coord. sphere | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| a The values of coordination sequences for the marked compounds are provided taking into account the uranyl-cation interactions. | |||||||
| Na[(UO2)(i-C3H7COO)3]·0.7H2O (I) | |||||||
| U1 | 3 | 5 | 9 | 13 | 18 | 25 | 40 |
| U2 | 2 | 2 | 4 | 8 | 15 | 21 | 29 |
| U3 | 3 | 5 | 9 | 13 | 19 | 25 | 38 |
| U4 | 3 | 5 | 8 | 12 | 19 | 25 | 36 |
| U5 | 1 | 1 | 2 | 4 | 8 | 12 | 19 |
| Cs[UO2(CH3COO)3] acetate ligand53a | |||||||
| U1 | 4 | 12 | 22 | 43 | 65 | 97 | 128 |
| U2 | 4 | 11 | 21 | 46 | 63 | 95 | 129 |
| Cs[UO2(C2H3COO)3] acrylate54 | |||||||
| U1 | 3 | 6 | 12 | 24 | 35 | 48 | 69 |
| Cs[UO2(C2H5COO)3] propionate46 | |||||||
| U1 | 3 | 6 | 12 | 24 | 35 | 48 | 69 |
| Cs[UO2(C3H5COO)3] crotonate ligand, layered structure48a | |||||||
| U1 | 4 | 12 | 18 | 24 | 30 | 36 | 42 |
| U2 | 4 | 12 | 18 | 24 | 30 | 36 | 42 |
| Cs[UO2(n-C3H7COO)3][UO2(n-C3H7COO)(OH)(H2O)] n-butyrate ligand, layered structure17a | |||||||
| U1 | 3 | 10 | 13 | 20 | 19 | 30 | 29 |
| U2 | 4 | 10 | 12 | 18 | 22 | 30 | 28 |
| Cs[UO2(n-C3H7COO)3] n-butyrate16 | |||||||
| U1 | 3 | 6 | 12 | 24 | 35 | 48 | 69 |
| Cs[UO2(i-C3H7COO)3] (II) isobutyrate | |||||||
| U1 | 3 | 6 | 12 | 24 | 35 | 48 | 69 |
| Highest theoretically possible number of neighbors10 | |||||||
| 3 | 6 | 12 | 24 | 48 | 96 | 192 | |
Interestingly, analogous to I, compound Na[(UO2)(n-C3H7COO)3]·0.25H2O15 with unbranched butyrate ions features only one out of four crystallographic sorts of sodium atoms with CN 5, leading to depolymerization, which is 25% of all the Na atoms. The here-studied compound I has three out of five Na atoms leading to depolymerization, which is 60% of all the Na atoms. As a result, the compound with a higher number of Na atoms leading to depolymerization has lower values of coordination sequences, although it features the ligands with the same number of carbon atoms in the hydrocarbon chains.18 Probably, this effect is explained by the decreased flexibility of the branched hydrocarbon chain of the isobutyrate ion: it does not allow the initially ideal cubic NaUO6 framework10 to be preserved without depolymerization after the ligands are placed in the voids of this framework.
The coordination sequences for the known cesium and uranyl complexes with aliphatic monocarboxylate ions are provided in Table 3. Let us remind, that in ideal AUO6 frameworks (A is a secondary metal atom), schematically represented in Fig. 4a, every R atom (R is either A or U) is connected with three R atoms via the R–O–R bridges and, thus, the R–(O–R–)N chains consecutively bifurcate on R atoms (see Fig. 4 and 6).10,16 This fact gives the highest theoretically possible number of neighbors equal to 3, 6, 12, 24, 48, 96, 192 and so on in the coordination spheres of R atoms in such AUO6 frameworks.10 Among seven listed Cs-containing compounds in Table 3, three compounds do not fit in the earlier described representation with the three-dimensional CsUO6 frameworks. Two of these compounds have layered structures (see Table 3) and, thus, no three-dimensional CsUO6 frameworks. Moreover, in the cases of compounds with acetate53 and crotonate48 ligands (see Table 3) the R–(O–R–)N chains bifurcate also on O atoms, but not only on R atoms (see the black and grey disks in Fig. 6), making it possible to have four neighbors in the first coordination sphere. In the case of the Cs[UO2(n-C3H7COO)3][UO2(n-C3H7COO)(OH)(H2O)] compound17 one out of two U atoms has CN 7 in contrast to all the other discussed U atoms with CN 8. The acetate, crotonate and n-butyrate hydroxo containing compounds also feature uranyl-cation UO22+–Cs+ interactions,48–50 which were taken into account for the calculation of coordination sequences, which makes the values of coordination sequences so high for the acetate complex (see Table 3 and black circles in Fig. 6). However, the layered structures of crotonate and n-butyrate hydroxo compounds prevent the values of coordination sequences from being as high as in the acetate containing complex (see Table 3).
The remaining four cesium and uranyl complexes with acrylate,54 propionate,46n-butyrate16 and isobutyrate (compound II) ligands from Table 3 can be represented with the concept of three-dimensional CsUO6 frameworks. Interestingly, they realize the same coordination sequences even though the size of the ligand changes considerably (see Table 3). Along with Na[UO2(CH3COO)3],55 K[UO2(C2H5COO)3]47 and K[UO2(C3H5COO)3]48 these are the only compounds that maintain the highest theoretically possible number of neighbors up to the fourth coordination sphere.10 In correspondence with the results in ref. 16 and 20, these crystal structures represent the optimal ratios between the sizes of the ligands and the pores of metal–oxygen frameworks, determined by the sizes of the secondary metal atoms.
Finally, it is worth noting, that sodium and cesium containing compounds with unbranched n-butyrate ions, analogous to I and II, possess lower symmetry than the branched ones: monoclinic Na[(UO2)(n-C3H7COO)3]·0.25H2O15vs. orthorhombic Na[(UO2)(i-C3H7COO)3]·0.7H2O (I) and orthorhombic Cs[UO2(n-C3H7COO)3]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16vs. cubic Cs[(UO2)(i-C3H7COO)3] (II).16 Thus, in spite of promoting depolymerization, branched isobutyrate ions contribute to maintaining a higher crystal system.
16vs. cubic Cs[(UO2)(i-C3H7COO)3] (II).16 Thus, in spite of promoting depolymerization, branched isobutyrate ions contribute to maintaining a higher crystal system.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Russian Science Foundation (project no. 17-73-10117). Synchrotron measurements were performed at the unique scientific facility Kurchatov Synchrotron Radiation Source supported by the Ministry of Education and Science of the Russian Federation (project code RFMEFI61917X0007). The contribution of the center for molecular composition studies of INEOS RAS is acknowledged.References
- K. P. Carter, M. Kalaj, R. G. Surbella, L. C. Ducati, J. Autschbach and C. L. Cahill, Chem. – Eur. J., 2017, 23, 15355–15369 CrossRef CAS PubMed.
- M. Dembowski, C. A. Colla, P. Yu, J. Qiu, J. E. S. Szymanowski, W. H. Casey and P. C. Burns, Inorg. Chem., 2017, 56, 9602–9608 CrossRef CAS PubMed.
- T. Zheng, Y. Gao, D. Gui, L. Chen, D. Sheng, J. Diwu, Z. Chai, T. E. Albrecht-Schmitt and S. Wang, Dalton Trans., 2016, 45, 9031–9035 RSC.
- P. Thuéry and J. Harrowfield, Dalton Trans., 2017, 46, 13677–13680 RSC.
- V. V. Gurzhiy, O. S. Tyumentseva, S. V. Krivovichev and I. G. Tananaev, J. Solid State Chem., 2017, 248, 126–133 CrossRef CAS.
- A. T. Kerr and C. L. Cahill, Cryst. Growth Des., 2011, 11, 5634–5641 CAS.
- G. R. Choppin, J. Radioanal. Nucl. Chem., 2007, 273, 695–703 CrossRef CAS.
- R. C. Ewing, W. Runde and T. E. Albrecht-Schmitt, MRS Bull., 2010, 35, 859–866 CrossRef CAS.
- S. De Carlo and J. R. Harris, Micron, 2011, 42, 117–131 CrossRef CAS PubMed.
- A. V. Savchenkov, A. V. Vologzhanina, L. B. Serezhkina, D. V. Pushkin and V. N. Serezhkin, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2013, 69, 721–726 CAS.
- A. V. Savchenkov, A. V. Vologzhanina, V. N. Serezhkin, D. V. Pushkin and L. B. Serezhkina, Crystallogr. Rep., 2014, 59, 190–195 CrossRef CAS.
- A. V. Savchenkov, V. V. Klepov, A. V. Vologzhanina, L. B. Serezhkina, D. V. Pushkin and V. N. Serezhkin, CrystEngComm, 2015, 17, 740–746 RSC.
- T. Loiseau, I. Mihalcea, N. Henry and C. Volkringer, Coord. Chem. Rev., 2014, 266–267, 69–109 CrossRef CAS.
- V. N. Serezhkin, A. V. Vologzhanina, L. B. Serezhkina, E. S. Smirnova, E. V. Grachova, P. V. Ostrova and M. Y. Antipin, Acta Crystallogr., Sect. B: Struct. Sci., 2009, 65, 45–53 CAS.
- D. V. Pushkin, A. V. Vologzhanina, L. B. Serezhkina, A. V. Savchenkov, A. A. Korlyukov and V. N. Serezhkin, Russ. J. Inorg. Chem., 2012, 57, 939–944 CrossRef CAS.
- A. V. Savchenkov, A. V. Vologzhanina, L. B. Serezhkina, D. V. Pushkin and V. N. Serezhkin, Polyhedron, 2015, 91, 68–72 CrossRef CAS.
- A. V. Vologzhanina, A. V. Savchenkov, A. O. Dmitrienko, A. A. Korlyukov, I. S. Bushmarinov, D. V. Pushkin and L. B. Serezhkina, J. Phys. Chem. A, 2014, 118, 9745–9752 CrossRef CAS PubMed.
- A. V. Savchenkov, A. V. Vologzhanina, L. B. Serezhkina, D. V. Pushkin and V. N. Serezhkin, Radiochemistry, 2013, 55, 466–471 CrossRef CAS.
- A. V. Savchenkov, E. V. Peresypkina, D. V. Pushkin, A. V. Virovets, L. B. Serezhkina and V. N. Serezhkin, J. Mol. Struct., 2014, 1074, 583–588 CrossRef CAS.
- V. N. Serezhkin, M. S. Grigoriev, A. R. Abdulmyanov, A. M. Fedoseev, A. V. Savchenkov and L. B. Serezhkina, Inorg. Chem., 2016, 55, 7688–7693 CrossRef CAS PubMed.
- Complex compounds of uranium, ed. I. I. Chernyaev, Program for Scientific Translations, Jerusalem, Israel, 1966 Search PubMed.
- I. A. Yuranov and K. M. Dunaeva, Koord. Khim., 1989, 15, 845–847 CAS.
- G. M. Sheldrick, SADABS, Program for empirical X-ray absorption correction, Bruker-Nonius, 1990 Search PubMed.
- P. Evans, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2006, 62, 72–82 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 CrossRef PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. a. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- Bruker, TOPAS 4.2 User Manual, Bruker AXS GmbH, Karlsruhe, Germany, 2009 Search PubMed.
- A. A. Coelho, J. Appl. Crystallogr., 2003, 36, 86–95 CAS.
- I. S. Bushmarinov, A. O. Dmitrienko, A. A. Korlyukov and M. Y. Antipin, J. Appl. Crystallogr., 2012, 45, 1187–1197 CAS.
- R. Cheary, A. Coelho and J. Cline, J. Res. Natl. Inst. Stand. Technol., 2004, 109, 1–26 CrossRef CAS PubMed.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- S. Parsons, H. D. Flack and T. Wagner, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2013, 69, 249–259 CAS.
- M. F. Ramos Moita, M. L. T. S. Duarte and R. Fausto, Spectrosc. Lett., 1994, 27, 1421–1430 CrossRef.
- R. M. Silverstein, F. X. Webster and D. Kiemle, Spectrometric Identification of Organic Compounds, Wiley, 7th edn, 2005 Search PubMed.
- A. Belsky, M. Hellenbrandt, V. L. Karen and P. Luksch, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 364–369 CrossRef.
- V. N. Serezhkin, M. S. Grigoriev, A. R. Abdulmyanov, A. M. Fedoseev, A. V. Savchenkov, S. Y. Stefanovich and L. B. Serezhkina, Inorg. Chem., 2017, 56, 7151–7160 CrossRef CAS PubMed.
- V. N. Serezhkin, M. O. Karasev and L. B. Serezhkina, Radiochemistry, 2013, 55, 137–146 CrossRef CAS.
- T. Steiner, Angew. Chem., Int. Ed., 2002, 41, 48–76 CrossRef CAS.
- G. R. Desiraju, Cryst. Growth Des., 2011, 11, 896–898 CAS.
- V. N. Serezhkin, A. G. Verevkin, O. P. Smirnov and V. P. Plakhtii, Russ. J. Inorg. Chem., 2010, 55, 1600–1606 CrossRef CAS.
- V. N. Serezhkin, N. A. Neklyudova and O. P. Smirnov, Russ. J. Inorg. Chem., 2012, 57, 864–869 CrossRef CAS.
- V. N. Serezhkin, Y. N. Mikhailov and Y. A. Buslaev, Russ. J. Inorg. Chem., 1997, 42, 1871–1910 Search PubMed.
- V. N. Serezhkin, in Structural Chemistry of Inorganic Actinide Compounds, ed. S. Krivovichev, P. Burns and I. Tananaev, Elsevier Science, 2007, pp. 31–65 Search PubMed.
- M. Kalaj, K. P. Carter, A. V. Savchenkov, M. M. Pyrch and C. L. Cahill, Inorg. Chem., 2017, 56, 9156–9168 CrossRef CAS PubMed.
- V. I. Burkov, V. E. Mistryukov, Y. N. Mikhailov and E. B. Chuklanova, Russ. J. Inorg. Chem., 1997, 42, 327–331 Search PubMed.
- L. B. Serezhkina, E. V. Peresypkina, A. V. Virovets, A. R. Abdul'myanov and V. N. Serezhkin, Radiochemistry, 2013, 55, 31–35 CrossRef CAS.
- A. V. Savchenkov, A. V. Vologzhanina, L. B. Serezhkina, D. V. Pushkin, S. Y. Stefanovich and V. N. Serezhkin, Z. Anorg. Allg. Chem., 2015, 641, 1182–1187 CrossRef CAS.
- S. Fortier and T. W. Hayton, Coord. Chem. Rev., 2010, 254, 197–214 CrossRef CAS.
- V. N. Serezhkin, G. V. Sidorenko, D. V. Pushkin and L. B. Serezhkina, Radiochemistry, 2014, 56, 115–133 CrossRef CAS.
- M. O'Keeffe, Z. Kristallogr. – Cryst. Mater., 1995, 210, 905–908 Search PubMed.
- N. Anisimova, R. Hoppe and M. Serafin, Z. Anorg. Allg. Chem., 1997, 623, 35–38 CrossRef.
- L. B. Serezhkina, A. V. Vologzhanina, V. V. Klepov and V. N. Serezhkin, Crystallogr. Rep., 2010, 55, 773–779 CrossRef CAS.
- V. V. Klepov, L. B. Serezhkina, A. V. Vologzhanina, D. V. Pushkin, O. A. Sergeeva, S. Y. Stefanovich and V. N. Serezhkin, Inorg. Chem. Commun., 2014, 46, 5–8 CrossRef CAS.
- A. Navaza, P. Charpin, D. Vigner and G. Heger, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47, 1842–1845 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1579816– 1579818. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt04042c |
| This journal is © The Royal Society of Chemistry 2018 |