Structure and nitrite reduction reactivity study of bio-inspired copper(i)–nitro complexes in steric and electronic considerations of tridentate nitrogen ligands†
Abstract
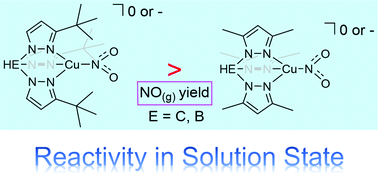
Two copper(I)–nitro complexes [Tpm3-tBuCu(NO2)] (1) and [(Ph3P)2N][Tp3-tBuCu(NO2)] (2), containing steric bulky neutral tris(3-tert-butylpyrazolyl)methane and anionic hydrotris(3-tert-butylpyrazolyl)borate ligands, have been synthesized and characterized. Complex 2 adopts a unique κ2-binding mode of Tp3-tBu around the copper(I)–nitro environment in the solid state and shows a four-coordinated tetrahedral geometry surrounded by a nitro and three pz3-tBu groups in solution. Both complexes 1 and 2 allow for the stoichiometric reduction of NO2− to NO with H+ addition. The results of this effort show that increasing steric bulk and electron donation properties on the nitrogen ancillary ligand will improve the nitrite reduction ability of the copper(I)–nitro model complexes.


 Please wait while we load your content...
Please wait while we load your content...